A skin patch to treat peanut allergies teaches the body to tolerate the nuts

Peanut allergies affect about a million children in the U.S., and most never outgrow them. Luckily, some promising remedies are in the works.
Ever since he was a baby, Sharon Wong’s son Brandon suffered from rashes, prolonged respiratory issues and vomiting. In 2006, as a young child, he was diagnosed with a severe peanut allergy.
"My son had a history of reacting to traces of peanuts in the air or in food,” says Wong, a food allergy advocate who runs a blog focusing on nut free recipes, cooking techniques and food allergy awareness. “Any participation in school activities, social events, or travel with his peanut allergy required a lot of preparation.”
Peanut allergies affect around a million children in the U.S. Most never outgrow the condition. The problem occurs when the immune system mistakenly views the proteins in peanuts as a threat and releases chemicals to counteract it. This can lead to digestive problems, hives and shortness of breath. For some, like Wong’s son, even exposure to trace amounts of peanuts could be life threatening. They go into anaphylactic shock and need to take a shot of adrenaline as soon as possible.
Typically, people with peanut allergies try to completely avoid them and carry an adrenaline autoinjector like an EpiPen in case of emergencies. This constant vigilance is very stressful, particularly for parents with young children.
“The search for a peanut allergy ‘cure’ has been a vigorous one,” says Claudia Gray, a pediatrician and allergist at Vincent Pallotti Hospital in Cape Town, South Africa. The closest thing to a solution so far, she says, is the process of desensitization, which exposes the patient to gradually increasing doses of peanut allergen to build up a tolerance. The most common type of desensitization is oral immunotherapy, where patients ingest small quantities of peanut powder. It has been effective but there is a risk of anaphylaxis since it involves swallowing the allergen.
"By the end of the trial, my son tolerated approximately 1.5 peanuts," Sharon Wong says.
DBV Technologies, a company based in Montrouge, France has created a skin patch to address this problem. The Viaskin Patch contains a much lower amount of peanut allergen than oral immunotherapy and delivers it through the skin to slowly increase tolerance. This decreases the risk of anaphylaxis.
Wong heard about the peanut patch and wanted her son to take part in an early phase 2 trial for 4-to-11-year-olds.
“We felt that participating in DBV’s peanut patch trial would give him the best chance at desensitization or at least increase his tolerance from a speck of peanut to a peanut,” Wong says. “The daily routine was quite simple, remove the old patch and then apply a new one. By the end of the trial, he tolerated approximately 1.5 peanuts.”
How it works
For DBV Technologies, it all began when pediatric gastroenterologist Pierre-Henri Benhamou teamed up with fellow professor of gastroenterology Christopher Dupont and his brother, engineer Bertrand Dupont. Together they created a more effective skin patch to detect when babies have allergies to cow's milk. Then they realized that the patch could actually be used to treat allergies by promoting tolerance. They decided to focus on peanut allergies first as the more dangerous.
The Viaskin patch utilizes the fact that the skin can promote tolerance to external stimuli. The skin is the body’s first defense. Controlling the extent of the immune response is crucial for the skin. So it has defense mechanisms against external stimuli and can promote tolerance.
The patch consists of an adhesive foam ring with a plastic film on top. A small amount of peanut protein is placed in the center. The adhesive ring is attached to the back of the patient's body. The peanut protein sits above the skin but does not directly touch it. As the patient sweats, water droplets on the inside of the film dissolve the peanut protein, which is then absorbed into the skin.
The peanut protein is then captured by skin cells called Langerhans cells. They play an important role in getting the immune system to tolerate certain external stimuli. Langerhans cells take the peanut protein to lymph nodes which activate T regulatory cells. T regulatory cells suppress the allergic response.
A different patch is applied to the skin every day to increase tolerance. It’s both easy to use and convenient.
“The DBV approach uses much smaller amounts than oral immunotherapy and works through the skin significantly reducing the risk of allergic reactions,” says Edwin H. Kim, the division chief of Pediatric Allergy and Immunology at the University of North Carolina, U.S., and one of the principal investigators of Viaskin’s clinical trials. “By not going through the mouth, the patch also avoids the taste and texture issues. Finally, the ability to apply a patch and immediately go about your day may be very attractive to very busy patients and families.”
Brandon Wong displaying origami figures he folded at an Origami Convention in 2022
Sharon Wong
Clinical trials
Results from DBV's phase 3 trial in children ages 1 to 3 show its potential. For a positive result, patients who could not tolerate 10 milligrams or less of peanut protein had to be able to manage 300 mg or more after 12 months. Toddlers who could already tolerate more than 10 mg needed to be able to manage 1000 mg or more. In the end, 67 percent of subjects using the Viaskin patch met the target as compared to 33 percent of patients taking the placebo dose.
“The Viaskin peanut patch has been studied in several clinical trials to date with promising results,” says Suzanne M. Barshow, assistant professor of medicine in allergy and asthma research at Stanford University School of Medicine in the U.S. “The data shows that it is safe and well-tolerated. Compared to oral immunotherapy, treatment with the patch results in fewer side effects but appears to be less effective in achieving desensitization.”
The primary reason the patch is less potent is that oral immunotherapy uses a larger amount of the allergen. Additionally, absorption of the peanut protein into the skin could be erratic.
Gray also highlights that there is some tradeoff between risk and efficacy.
“The peanut patch is an exciting advance but not as effective as the oral route,” Gray says. “For those patients who are very sensitive to orally ingested peanut in oral immunotherapy or have an aversion to oral peanut, it has a use. So, essentially, the form of immunotherapy will have to be tailored to each patient.” Having different forms such as the Viaskin patch which is applied to the skin or pills that patients can swallow or dissolve under the tongue is helpful.
The hope is that the patch’s efficacy will increase over time. The team is currently running a follow-up trial, where the same patients continue using the patch.
“It is a very important study to show whether the benefit achieved after 12 months on the patch stays stable or hopefully continues to grow with longer duration,” says Kim, who is an investigator in this follow-up trial.
"My son now attends university in Massachusetts, lives on-campus, and eats dorm food. He has so much more freedom," Wong says.
The team is further ahead in the phase 3 follow-up trial for 4-to-11-year-olds. The initial phase 3 trial was not as successful as the trial for kids between one and three. The patch enabled patients to tolerate more peanuts but there was not a significant enough difference compared to the placebo group to be definitive. The follow-up trial showed greater potency. It suggests that the longer patients are on the patch, the stronger its effects.
They’re also testing if making the patch bigger, changing the shape and extending the minimum time it’s worn can improve its benefits in a trial for a new group of 4-to-11 year-olds.
The future
DBV Technologies is using the skin patch to treat cow’s milk allergies in children ages 1 to 17. They’re currently in phase 2 trials.
As for the peanut allergy trials in toddlers, the hope is to see more efficacy soon.
For Wong’s son who took part in the earlier phase 2 trial for 4-to-11-year-olds, the patch has transformed his life.
“My son continues to maintain his peanut tolerance and is not affected by peanut dust in the air or cross-contact,” Wong says. ”He attends university in Massachusetts, lives on-campus, and eats dorm food. He still carries an EpiPen but has so much more freedom than before his clinical trial. We will always be grateful.”
An emerging technology called in vitro gametogenesis would allow babies to be made from skin cells.
[Ed. Note: This is the third episode in our Moonshot series, which will explore four cutting-edge scientific developments that stand to fundamentally transform our world.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
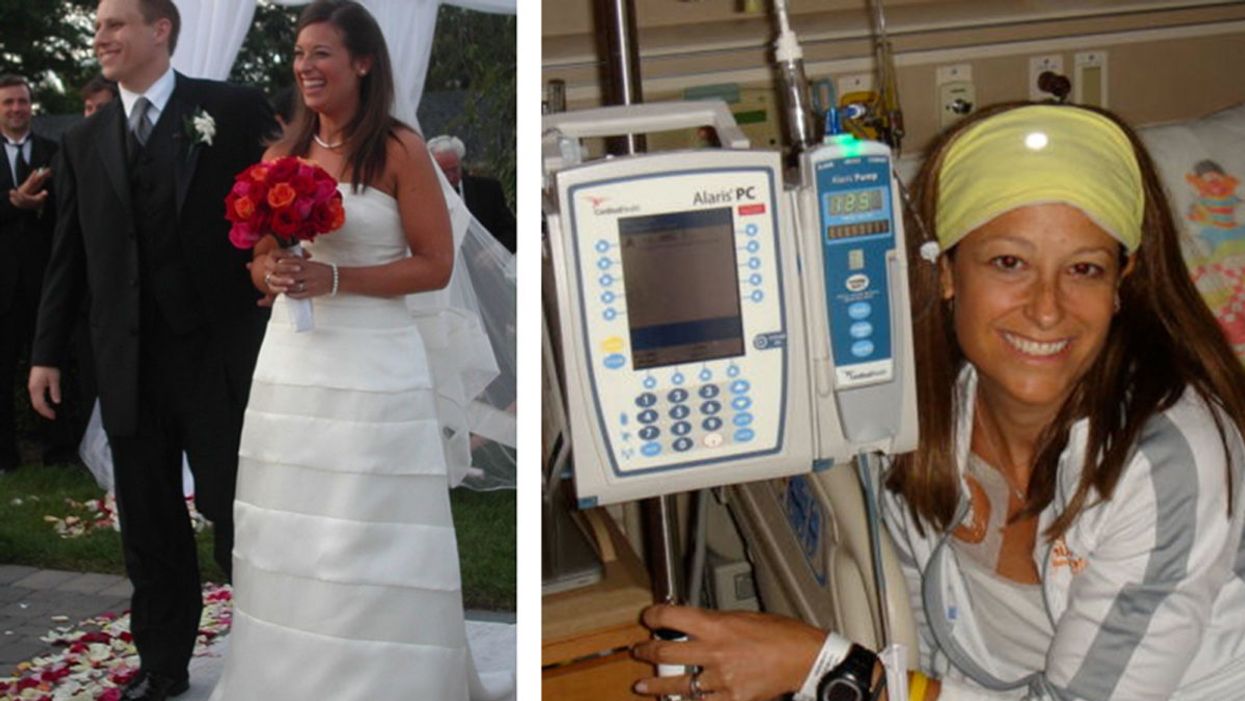
My Wife's Fight Against Cancer Inspired 38,000 People to Raise Millions for Research
David and Jen on their wedding day, and Jen undergoing treatment for her rare sarcoma.
It was 15 years ago this month, but I'll never forget those words. When my wife Jen and I asked her oncologist about our plans to start a family, he calmly replied, "Well, I wouldn't do so unless Dave is prepared to be a single father."
About 50 percent of all people with cancer have a rare type, like the one Jen was fighting.
Time stood still. The danger crystalized — we were in a battle for my beautiful bride's life, and the odds were not in our favor.
We felt every emotion expected. Anger, sadness, confusion, frustration, and especially fear. But we made a very intentional choice to take that fear, put it to the side, and do everything we could to live our lives together to the fullest.
We focused first on Jen's health and learned everything we could about MFH Sarcoma. I was with her every step of the way — for hundreds of medical appointments, six intense surgeries, and twenty different types of chemotherapy. During such a challenging time, our choice to reject fear allowed us to live our best lives. Our careers blossomed, we enjoyed several international vacations, and Jen inspired thousands of fellow patients through her blog and speeches.
When we researched treatment options we learned that Jen was not alone. About 50 percent of all people with cancer have a rare type, like the one Jen was fighting. However, rare cancers don't get the funding they desperately need so effective treatment options are hard to find. The lack of funding felt unfair — and urgent. We didn't worry about everything that can go wrong when starting a new venture. Instead, we jumped in head first and convinced a small group of friends and family to ride stationary bikes with us to raise money for rare cancer research.

Jen Goodman Linn, riding a stationary bike for Cycle for Survival.
(Courtesy David Linn)
From those humble beginnings, Cycle for Survival grew steadily. After starting from scratch, Jen and I ran Cycle for Survival on our own for two years. We quickly realized that if we wanted to help as many people as possible, we needed the best partners. In 2009, we agreed that Memorial Sloan Kettering Cancer Center would take over the ownership of Cycle for Survival and Equinox officially became the Founding Partner. Flash forward to today, and Cycle for Survival has raised more than $220 million! I'm proud that 100% of every donation, yes every penny, goes directly into life-saving rare cancer research within six months of the annual indoor cycling events, which now take place in 17 cities nationwide.
While Cycle for Survival's trajectory was heading straight up, Jen's health struggle was devastatingly swinging up and down. With her incredible spirit and tenacity, Jen would beat the cancer through chemo and surgery, but then it would frustratingly come back again and again. After going into remission six times, it returned with such a vengeance in 2011 that even the world's leading doctors were forced to say, "I'm sorry, there's nothing more we can do."
Those were the most difficult words I've ever heard, by far. I hope no other family has to hear these crushing words.
When Jen died soon after, I didn't know what would happen to me, to my life, and to Cycle for Survival. I do remember making two very important choices at the time. First, I chose to get out of bed and put one foot in front of the other. It wasn't easy. Tears, pain, and grief would hit at any hour of the day or night. I did have a great support network of family and friends who kept me moving forward. One friend in particular changed the route of her morning runs so that I would join her and start getting back to exercising.
My second key choice was to stay involved with Cycle for Survival. At times, it was an excruciatingly difficult decision because I felt the depth of my loss each and every time I stepped into one of the events. However, it was also rewarding and energizing because I could see firsthand how many people it was helping, even though it was too late for Jen.
I began to travel across the country with the Cycle for Survival staff. My hope was to spread the word about rare cancers; along the way I met a lot of wonderful people who shared their stories with me. What I soon realized is that each of us faces obstacles in our lives. For me, it was losing the person who I wanted to spend my life with. For others, it might be challenges with their kids or in their professional lives. The common theme is that we don't have control over the fact that we have to face these challenges. But the biggest lesson I've learned is that we very much do have a choice in how we react.
I made the choice to do everything I can to help rare cancer patients and their families and it has been transformative and healing for me. The small group who rode in the first Cycle for Survival event has grown into a powerful movement of nearly 40,000 riders making a real difference. If Jen were diagnosed today, there are new treatments available– including genomic sequencing, targeted therapies, and immunotherapies – that could help her. Those weren't even options a short time ago. That's the result of funding research.

A recent Cycle for Survival event shows the passion and power of the community.
(Courtesy David Linn)
I also want to share one more choice I made. Remember that friend who changed the route of her morning runs so I could start exercising after Jen died? Well, over the years friendship grew into love, and we're now building a home together and can't wait to see what the future holds for us.
So with all that in mind I ask – when you face those inevitable challenges in your life, how will you choose to react? Remember that even in the midst of hopelessness, you can find choices. Those will be the decisions that define and guide you.