Forget Farm-to-Table: Lab-to-Table Fresh Fish Is Making Waves
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

A conventionally sourced sea bass from a fishery.
Ever wonder why you've never heard of wild-caught organic fish? It's because there's no way to certify a food that has a mysterious history. Mike Selden, a 26-year-old biochemist with an animal lover's heart and an entrepreneur's mind, decided there must be better way to consume one of our planet's primary sources of animal protein. A way that would eliminate the need to kill billions of fish per year while also producing toxin-free, cheap, delicious fish meat for your dinner table. Enter Finless Foods, a young startup with a bold vision. Selden took time out of chauffeuring fish carcasses around San Francisco (no joke!) to share his journey with LeapsMag.
What is the biggest problem with the way fish is consumed today?
There are a lot of problems ranging from metals to animal welfare to human health. Technology is solving those problems at the same time. You've got extreme over fishing, which is collapsing ocean ecosystems and removing populations of fish that are traditionally used as food sources in developing nations.
In terms of animal welfare, fish are killed in massive numbers, billions a year. Even if people don't care too much about that, we want to give them another option.
In terms of health, which I think for most people is the most convincing argument, current fish have mercury and plastic in them. And if you're getting that fish from a farm, you will also have high levels of antibiotics and growth hormones if you're getting it from outside the U.S. What we're doing is producing fish that doesn't have any of those contaminants.
What gave you the idea to start a company around lab-grown fish?
I studied biochemistry and molecular biology at UMass Amherst, traditionally an agricultural school out in the woods of Massachusetts. I have always been an environmental activist and cared about animals. I thought, animal agriculture is so incredibly inefficient, what could be done to change it?
"The worst way you can possibly make a hamburger is with a cow."
Agriculture is a system of inputs and outputs, the inputs being feed and the outputs being meat – so why are we wasting all of this input on outputs we don't care about? Why are we creating these animals that waste all this energy through sitting around, moving around, having a heartbeat, blinking? All of this uses energy and that's valuable input.
The worst way you can possibly make a hamburger is with a cow. It's an awful transfer of energy: you have to feed it many times its own weight in food that could have fed other people or other things.
In February, I got funding from Indie Bio, a startup accelerator for synthetic biology, and moved out to San Francisco with my co-founder Brian Wyrwas. We started working in our lab in March. We're the newest company in the space.
Walk me through the process of creating edible fish in the lab. Do you have to catch a real live fish first and get their cells?
We have a deal with the Aquarium of the Bay, and whenever a fish dies, they call me, I get in a zip car, drive over, and bring the fish back to the lab, where Brian cultures it up into a cell culture. We do use real, high-quality fish stock. From there, we get the cells going in a bioreactor in a suspension culture, grow them into large quantities, and then bring them out to differentiate them into the cells people want to eat—the muscle and fat tissue. Then we formulate it and bring it to people's tables.
How long does the whole process take from the phone call about the fish dying to the food on the table?
There are two different processes: One is a research process, getting the initial cells and engineering them to be what we're looking for.
The other is a production process – we have a cell line ready and need to grow it out. That timing depends on how big of a facility we have. Since we're working with cell division: If you have 1 cell, in 24 hours, you'll have two cells. Let's say you have 1 ton of cells, in 24 hours you'll have two tons of cells.
"We want to give people the wholesome food they are used to in a healthier setting."
How are you looking to scale this process?
We're trying to find a middle ground between efficiency and local distribution. Organic farming is hilariously bad for the environment and horrifyingly inefficient, but on the other hand, industrial agriculture requires lots of transport, which is also bad for the environment. We're looking to create regionally distributed facilities which don't require a lot of transit, so people can have fresh fish even extremely far inland.
What kinds of fish are you "cooking"?
Our first product will be Bluefin tuna. It's a high-quality fish with high demand and it's also a conservation issue. We also currently have a culture going with Branzino, European sea bass, that we're really happy with.
There's a concept in science called a model organism – one that is extremely well studied and understood. Like the fruit fly, for example. For fish, it's the zebra fish, which is used for genetic research, but no one eats it. It's tiny, so we started by thinking: what fish do people eat that is also close evolutionarily to the zebra fish? We came up with carp, even though it's not too widely eaten.
But our process is very species agnostic. We've done work in trout, salmon, goldfish. Any fish with a dorsal fin works with our process. We tried a wolf eel but it didn't work. Eels are pretty far evolutionarily from fish, so we dropped that one.

From left to right, Ron Shigeta (IndieBio), Brian Wyrwas (Finless Foods), Amy Fleming (The Guardian), and Jihyun Kim (Finless Foods) tasting the first ever clean carp croquettes.
(Courtesy Mike Selden)
Why fish as opposed to, say, a cow?
Scientifically, there are a lot of advantages. Fish have a simpler structure than land animals. A fillet from a cow has complex marbling going on between the fat and muscle. When it's fish, like sashimi, it's in layers of muscle and fat. So it's simpler to build, plus fish are cold-blooded, so because they breathe underwater, our equipment needs less complexity. We don't need a CO2 line and we don't need to culture our cells at 37 degrees Celsius. We culture them at room temperature.
It's also easier to get to market since there's much higher value. Chicken in the last year was $3.84 per pound in America, whereas Bluefin tuna is between $100 and $1200 a pound. Because this is about dropping cost, we can get to market faster and give investors a better value proposition.
What's also cool is that something like Bluefin tuna is something many people haven't had the opportunity to eat. We can get these down in cost until there is price parity with any cheap conventional fish. We want to give people a choice between buying something like albacore tuna in a can –with mercury and plastic– or high-quality tuna without any contaminants for the same price.
Do you shape them like fish fillets to help the consumer overcome whatever discomfort they might feel about eating a bunch of lab-grown cells?
Yeah, people want to continue eating food they are eating, and that's fine. We want to give people a better option. We don't want to give them something weird and out there. We want to give them the wholesome food they are used to in a healthier setting that also solves some environmental issues.
How about the taste? Have you done any blind side-by-side tests with the real thing and your version?
Not blind taste tests. But we have been tasting it, and it is firmly fish. I even tried leaving it outside of the fridge – and man, that tasted like spoiled fish.
We want it to have the exact same properties as real fish. We don't want people to have to learn how to cook with it. We want them to just bring it into their homes and eat it exactly like they were doing before, but better.
What you're growing isn't the whole fish, right? It is not an actual organism?
Right, we're only growing muscle cells. It doesn't know where it is. There is no brain, nervous system, or pain receptors.
Are you the only people in this lab-grown food space working on fish?
We're the only ones doing fish so far. Other companies are doing chicken, duck, egg white, milk, gelatin, leather, and beef.
Are people generally weirded out by sci-fi lab food, or intrigued?
It's been very positive. When people sit down and talk to us, they realize it's not some crazed money grab or some weird Ted talk, it's real activists using real science trying to solve real problems. Sure, there will be some pushback from people who don't understand it, and that's fine.
When can I expect to see Finless Food at my local Whole Foods?
We plan on being in restaurants in two years, and grocery stores in four years.
What about people who aren't big fans of fish in the first place? Like those who don't eat sushi, because consuming something raw with an unknown history isn't very appetizing.
There are too many examples of food poisoning because fish are in a less clean environment than they should be, swimming around in their own fecal matter, and being doused in antibiotics so their diseases don't transmit. It's a bit of a mess. That's why as an industry, we're calling this clean meat. Fish is a healthy thing, or at least it should be, with Omega 3 and 6, and DHA. This is a way for people to continue getting those nutrients without any of the questions of where it came from. For people who are skeptical of fish, we invite you to dive in.

Brian Wyrwas, Co-Founder & CSO, and Mike Selden, Co-Founder & CEO
(Courtesy Mike Selden)
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
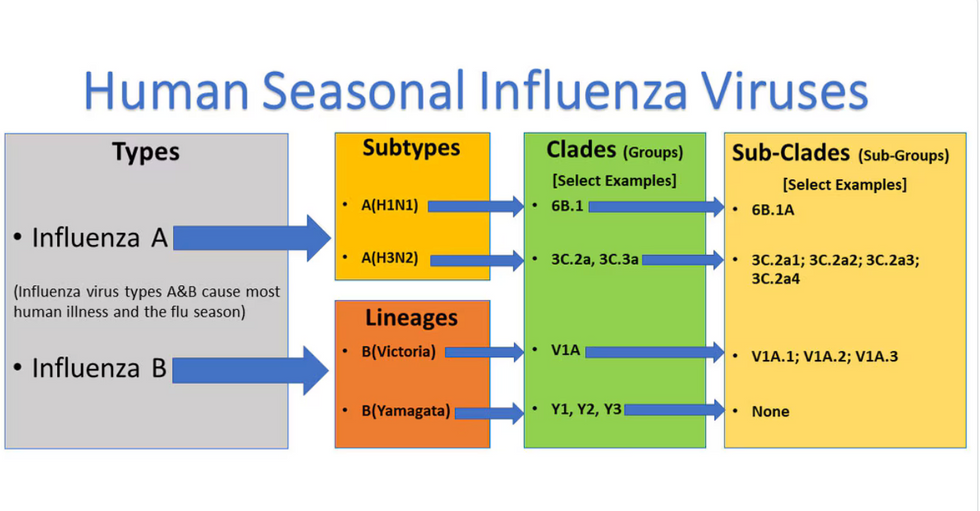
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

