With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
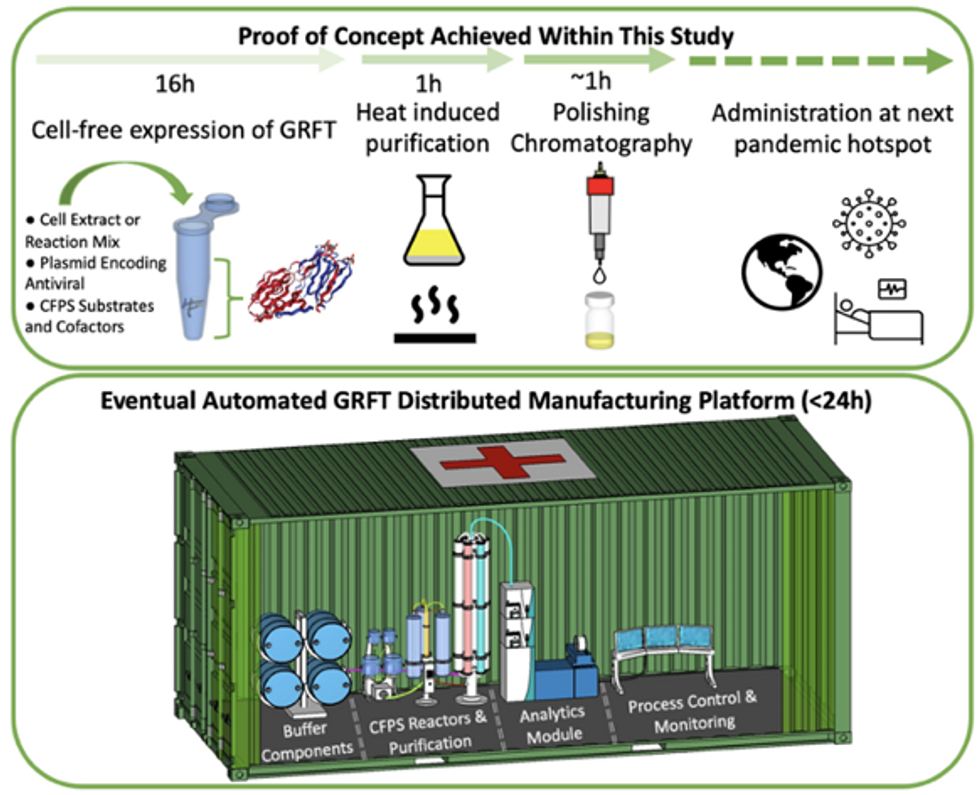
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Your phone could show if a bridge is about to collapse
Researchers have tested a new smartphone app that could gather data about whether bridges are "structurally deficient" —a low-cost citizen-scientist alternative to current methods that are expensive and complex.
In summer 2017, Thomas Matarazzo, then a postdoctoral researcher at the Massachusetts Institute of Technology, landed in San Francisco with a colleague. They rented two cars, drove up to the Golden Gate bridge, timing it to the city’s rush hour, and rode over to the other side in heavy traffic. Once they reached the other end, they turned around and did it again. And again. And again.
“I drove over that bridge 100 times over five days, back and forth,” says Matarazzo, now an associate director of High-Performance Computing in the Center for Innovation in Engineering at the United States Military Academy, West Point. “It was surprisingly stressful, I never anticipated that. I had to maintain the speed of about 30 miles an hour when the speed limit is 45. I felt bad for everybody behind me.”
Matarazzo had to drive slowly because the quality of data they were collecting depended on it. The pair was designing and testing a new smartphone app that could gather data about the bridge’s structural integrity—a low-cost citizen-scientist alternative to the current industrial methods, which aren’t always possible, partly because they’re expensive and complex. In the era of aging infrastructure, when some bridges in the United States and other countries are structurally unsound to the point of collapsing, such an app could inform authorities about the need for urgent repairs, or at least prompt closing the most dangerous structures.
There are 619,588 bridges in the U.S., and some of them are very old. For example, the Benjamin Franklin Bridge connecting Philadelphia to Camden, N.J., is 96-years-old while the Brooklyn Bridge is 153. So it’s hardly surprising that many could use some upgrades. “In the U.S., a lot of them were built in the post-World War II period to accommodate the surge of motorization,” says Carlo Ratti, architect and engineer who directs the Senseable City Lab at Massachusetts Institute of Technology. “They are beginning to reach the end of their life.”
According to the 2022 American Road & Transportation Builders Association’s report, one in three U.S. bridges needs repair or replacement. The Department of Transportation (DOT) National Bridge Inventory (NBI) database reveals concerning numbers. Thirty-six percent of U.S. bridges need repair work and over 78,000 bridges should be replaced. More than 43,500 bridges are rated in poor condition and classified as “structurally deficient” – an alarming description. Yet, people drive over them 167.5 million times a day. The Pittsburgh bridge which collapsed in January this year—only hours before President Biden arrived to discuss the new infrastructure law—was on the “poor” rating list.
Assessing the structural integrity of a bridge is not an easy endeavor. Most of the time, these are visual inspections, Matarazzo explains. Engineers check cracks, rust and other signs of wear and tear. They also check for wildlife—birds which may build nests or even small animals that make homes inside the bridge structures, which can slowly chip at the structure. However, visual inspections may not tell the whole story. A more sophisticated and significantly more expensive inspection requires placing special sensors on the bridge that essentially listen to how the bridge vibrates.
“Some bridges can afford expensive sensors to do the job, but that comes at a very high cost—hundreds of thousands of dollars per bridge per year,” Ratti says.
We may think of bridges as immovable steel and concrete monoliths, but they naturally vibrate, oscillating slightly. That movement can be influenced by the traffic that passes over them, and even by wind. Bridges of different types vibrate differently—some have longer vibrational frequencies and others shorter ones. A good way to visualize this phenomenon is to place a ruler over the edge of a desk and flick it slightly. If the ruler protrudes far off the desk, it will vibrate slowly. But if you shorten the end that hangs off, it will vibrate much faster. It works similarly with bridges, except there are more factors at play, including not only the length, but also the design and the materials used.
The long suspension bridges such as the Golden Gate or Verrazano Narrows, which hang on a series of cables, are more flexible, and their vibration amplitudes are longer. The Golden Gate Bridge can vibrate at 0.106 Hertz, where one Hertz is one oscillation per second. “Think about standing on the bridge for about 10 seconds—that's how long it takes for it to move all the way up and all the way down in one oscillation,” Matarazzo says.
On the contrary, the concrete span bridges that rest on multiple columns like Brooklyn Bridge or Manhattan Bridge, are “stiffer” and have greater vibrational frequencies. A concrete bridge can have a frequency of 10 Hertz, moving 10 times in one second—like that shorter stretch of a ruler.
The special devices that can pick up and record these vibrations over time are called accelerometers. A network of these devices for each bridge can cost $20,000 to $50,000, and more—and require trained personnel to place them. The sensors also must stay on the bridge for some time to establish what’s a healthy vibrational baseline for a given bridge. Maintaining them adds to the cost. “Some bridges can afford expensive sensors to do the job, but that comes at a very high cost—hundreds of thousands of dollars per bridge per year,” Ratti says.
Making sense of the readouts they gather is another challenge, which requires a high level of technical expertise. “You generally need somebody, some type of expert capable of doing the analysis to translate that data into information,” says Matarazzo, which ticks up the price, so doing visual inspections often proves to be a more economical choice for state-level DOTs with tight budgets. “The existing systems work well, but have downsides,” Ratti says. The team thought the old method could use some modernizing.
Smartphones, which are carried by millions of people, contain dozens of sensors, including the accelerometers capable of picking up the bridges’ vibrations. That’s why Matarazzo and his colleague drove over the bridge 100 times—they were trying to pick up enough data. Timing it to rush hour supported that goal because traffic caused more “excitation,” Matarazzo explains. “Excitation is a big word we use when we talk about what drives the vibration,” he says. “When there's a lot of traffic, there's more excitation and more vibration.” They also collaborated with Uber, whose drivers made 72 trips across the bridge to gather data in different cars.
The next step was to clean the data from “noise”—various vibrations that weren’t relevant to the bridge but came from the cars themselves. “It could be jumps in speed, it could be potholes, it could be a bunch of other things," Matarazzo says. But as the team gathered more data, it became easier to tell the bridge vibrational frequencies from all others because the noises generated by cars, traffic and other things tend to “cancel out.”
The team specifically picked the Golden Gate bridge because the civil structural engineering community had studied it extensively over the years and collected a host of vibrational data, using traditional sensors. When the researchers compared their app-collected frequencies with those gathered by 240 accelerometers formerly placed on the Golden Gate, the results were the same—the data from the phones converged with that from the bridge’s sensors. The smartphone-collected data were just as good as those from industry devices.
The study authors estimate that officials could use crowdsourced data to make key improvements that would help new bridges to last about 14 years longer.
The team also tested their method on a different type of bridge—not a suspension one like the Golden Gate, but a concrete span bridge in Ciampino, Italy. There they compared 280 car trips over the bridge to the six sensors that had been placed on the bridge for seven months. The results were slightly less matching, but a larger volume of trips would fix the divergence, the researchers wrote in their study, titled Crowdsourcing bridge dynamic monitoring with smartphone vehicle trips, published last month in Nature Communications Engineering.
Although the smartphones proved effective, the app is not quite ready to be rolled out commercially for people to start using. “It is still a pilot version,” so there’s room for improvement, says Ratti, who co-authored the study. “But on a more optimistic note, it has really low barriers to entry—all you need is smartphones on cars—so that makes the system easy to reach a global audience.” And the study authors estimate that the use of crowdsourced data would result in a new bridge lasting about 14 years longer.
Matarazzo hopes that the app could be eventually accessible for your average citizen scientist to collect the data and supply it to their local transportation authorities. “I hope that this idea can spark a different type of relationship with infrastructure where people think about the data they're collecting as some type of contribution or investment into their communities,” he says. “So that they can help their own department of transportation, their own municipality to support that bridge and keep it maintained better, longer and safer.”
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
The Friday Five: Sugar could help catch cancer early
In this week's Friday Five, catching cancer early could depend on sugar, how to boost memory in a flash, a tiny sandwich cake could help the heart, and meet the top banana in the fight against Covid.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- Catching cancer early could depend on sugar
- How to boost memory in a flash
- This is your brain on books
- A tiny sandwich cake could help the heart
- Meet the top banana for fighting Covid variants

