From Crap to Cure: The Story of Fecal Transplants

Meg Newman, who suffered from C. difficile, underwent a fecal transplant that helped restore her to health.
C. difficile had Meg Newman's number; it had struck her 18 different times beginning in 1985. The bacterial infection takes over the gut bringing explosive diarrhea, dehydration, weight loss, and at its worst depletes blood platelets. It causes nearly 30,000 deaths each year in the U.S. alone.
"I was one sick puppy as that point and literally three days after the transplant I was doing pretty well, day four even better."
Meg knew these statistics not just from personal experience but also because she was a doctor at San Francisco General Hospital. Antibiotics had sometimes helped to treat the infection, but it never quite seemed to go away. Now, "It felt like part of my colon was sort of sliding off as I had the bowel movement." On her worst day she counted 33 bowel movements. It was 2005 and she knew she was at the end of her rope.
Medical training had taught Meg to look at the data. So when antibiotics failed, she searched the literature for other options. One was a seemingly off-the-wall treatment called fecal transplants, which essentially gives poop from a healthy person to one who is sick.
Its roots stretch back to "yellow soup" used to treat dysentery in China nearly two thousand years ago, in which ancient Chinese treaters would combine stool with liquid, mash it up, and administer it. The approach also is commonly used in veterinary medicine today. However, there were only about three papers on its use in humans in the medical literature at that time, she recalls. Still, the logic of the intervention appealed to her.
The gut microbiome as a concept and even a word were not widely known fifteen years ago. But the idea that the microbial community in her gut was in disarray, and a transplant of organisms from a healthy gut might help restore a more normal ecology made sense. And besides, the failure of standard medicine left her few options.
Meg spoke with a colleague, gastroenterologist Neil Stollman, about a possible fecal microbial transplant (FMT). Only a handful of doctors in the U.S. had ever done the procedure; Stollman had tried it just once before. After conversation with Newman, he agreed to do it.
They decided on Meg's partner Sherry as the donor. "I try very hard to use intimate sexual partners as the donor," explains Stollman. The reason is to reduce disease risk: "The logic there is pretty straightforward. If you have unprotected sex with this individual, in a monogamous way for a period of time, you have assumed pretty much any infectious risk," like hepatitis, HIV, and syphilis, he says. Other donors would be screened using the same criteria used to screen blood donations, plus screening for parasites that can live in stool but not blood.
The procedure
Martini aficionados fall into two camps, fans of shaken or stirred. In the early days the options for producing of fecal transplants were a blender or hand shaken. Stollman took the hands-on approach, mixing Sherry's fecal donation with saline to create "a milkshake in essence." He filtered it several times through gauze to get out the lumps.
Then he inserted a colonoscope, a long flexible tube, through the anus into Meg's colon. Generally a camera goes through the tube to look for polyps and cancers, while other tools can snip off polyps and retrieve tissue samples. Today he used it to insert the fecal "milkshake" as high up the colon as he could go. Imodium and bed rest were the final pieces. It works about 90 percent of the time today.
Meg went home with fingers crossed. "And within about two weeks things just slowed down; the diarrhea just stopped. I felt better so my appetite was better." The tide had turned, though it would take months to slowly repair the toll taken on her body from disease and antibiotics.
Then in 2011 another serious medical challenge required heavy use of antibiotics and Meg's C. difficile came roaring back; she needed a second FMT. Sherry had a bad sinus infection and had been on antibiotics, so that ruled her out as a donor. Red, Meg's godson, volunteered. He was twenty-one and had little or no exposure to antibiotics, which can harm friendly bacteria living in the gut.
"I was one sick puppy as that point," Meg recalls, "and literally three days after the transplant [from Red] I was doing pretty well, day four even better. It was unbelievable." It illustrated that donors are not the same, and that while an intimate partner may be the safest option, it also may not be the most efficacious donation in terms of providing missing parts of the microbial ecosystem.
Going mainstream
By then, FMTs were starting to come out of the shadows as more than just a medical oddity. One gigantic milestone in changing perceptions was a Dutch study on using the procedure to treat C. difficile that was published in January 2013 in the New England Journal of Medicine. "That was the first trial where people said, look this isn't voodoo. This wasn't made up; it really worked," says Colleen Kelly, another pioneer in using FMTs to treat C. difficile and a researcher at Brown University. A single dose was successful more than 80 percent of the time in resolving disease in patients who had failed multiple regimens of antibiotics.
Charlatans pounced on the growing interest in the microbiome, promoting FMT as a cure for all sorts of ailments for which there was no scientific evidence. The FDA stepped in, announcing it would regulate the procedure as a drug, and essentially banned use of FMTs until it wrote regulations. But the outcry from physicians and patients was so great it was forced to retreat and has allowed continued use in treating C. difficile albeit on an interim regulatory basis that has continued since 2013.
Another major change was the emergence of stool banks, modeled on blood banks. The most widely know is OpenBiome, established in 2012 as a nonprofit by young researchers at Harvard and MIT. It aimed to standardize donation of stool and screening for disease, and package them in frozen form for colonoscopic delivery, or pill form. It greatly simplified the process and more doctors became willing to use FMTs to treat C. difficile. Today, some gastroenterologists specialize in administering the transplants as a feature of their practice.
To be sure, there have been some setbacks, including a transplant between family members where the recipient became obese, likely in part because of bacteria in the material she received. The same thing has occurred in studies in mice. And last year, an elderly man died from a toxic strain of E. coli that was in material provided by a stool bank. That caused the FDA to add that pathogen to the list of those one must screen for in products designed for use as fecal transplants.
Cost remains an issue. OpenBiome charges $1500-$2000 per transplant dose, depending on whether a frozen or pill form of the product is used. And that is likely to go up as the FDA increases the number of diseases that must be screened for, such as the virus that causes COVID-19, which is present in feces and likely can be transmitted through feces. Most insurance companies do not cover FMTs because no product has been formally approved for use by the FDA.
One of the greatest treatment challenges is making the correct diagnosis, says physician Robin Patel, who initially treated patients at the Mayo Clinic in Rochester, Minnesota but now spends most of her time there developing new diagnostics. Many things can cause diarrhea and the simple presence of the organism does not mean that C. difficile is causing it. In addition, many people are colonized with the bug but never develop symptoms of the disease.
Patel used the expensive tool of whole genome sequencing to look in great detail at C. difficile in patients who were treated with antibiotics for the infection and had recurrent diarrhea. "Some of them, as you might predict, were getting their symptoms with the same exact strain [of C. difficile] but others were not, it was a different strain," suggesting that they had been reinfected.
If it is a different strain, you might want to try antibiotics, she says, but if the same strain is present, then you might want to try a different approach such as FMT. Whole genome sequencing is still too slow and expensive to use in regularly treating patients today, but Patel hopes to develop a rapid, cost-effective test to help doctors make those types of decisions.
Biotech companies are trying to develop alternatives to poop as a source for transplant to treat C. difficile. They are picking and choosing different bacteria that they can grow and then combine into a product, to varying degrees of success, but none have yet crossed the finish line of FDA approval.
"I think [the future of FMTs] is going to be targeted, even custom-made."
The FDA would like such a product because it is used to dealing with small molecule drugs that are standardized and produced in batches. Companies are pursing it because, as Kelly says, they are like sharks "smelling money in the water." Approval of such a product might cause the FDA to shut down existing stool banks that now exist in a regulatory limbo, leaving the company with a monopoly of exclusive rights to the treatment.
Back when Meg received her first fecal transplant, the procedure was so obscure that the guidelines for treating C. difficile put out by the American College of Gastroenterology didn't even mention FMT. The procedure crept into the 2013 revision of those guidelines as a treatment of last resort. Guidance under review for release later this year or early next year will ease use further but stop short of making it a first option.
Stollman imagines a future holy grail in treating C. difficile: "You give me a stool specimen and I run it through a scanner that tells me you have too much of this and too little of that. I have a sense of what normal [microbial composition of the gut] should be and add some of this and subtract some of that. Maybe I even give you some antibiotics to get rid of some of the bad guys, give you some probiotics. I think it is going to be targeted, even custom-made."
His complete vision for treating C. difficile won't arrive tomorrow, but given how rapidly FMTs have become part of medicine, it is likely to arrive in pieces and more quickly than one might think.
About five years ago Meg discovered she had an antibody deficiency that contributed to her health problems, including vulnerability to C. difficile. She began supplementation with immunoglobulin and "that has made a huge difference in my health. It is just unbelievable how much better I am." She is grateful that fecal transplants gave her the time to figure that out. She believes "there's every reason to consider it [FMT] as a first-line treatment and do the studies, ASAP."
The Friday Five: How to exercise for cancer prevention
How to exercise for cancer prevention. Plus, a device that brings relief to back pain, ingredients for reducing Alzheimer's risk, the world's oldest disease could make you young again, and more.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- How to exercise for cancer prevention
- A device that brings relief to back pain
- Ingredients for reducing Alzheimer's risk
- Is the world's oldest disease the fountain of youth?
- Scared of crossing bridges? Your phone can help
New approach to brain health is sparking memories
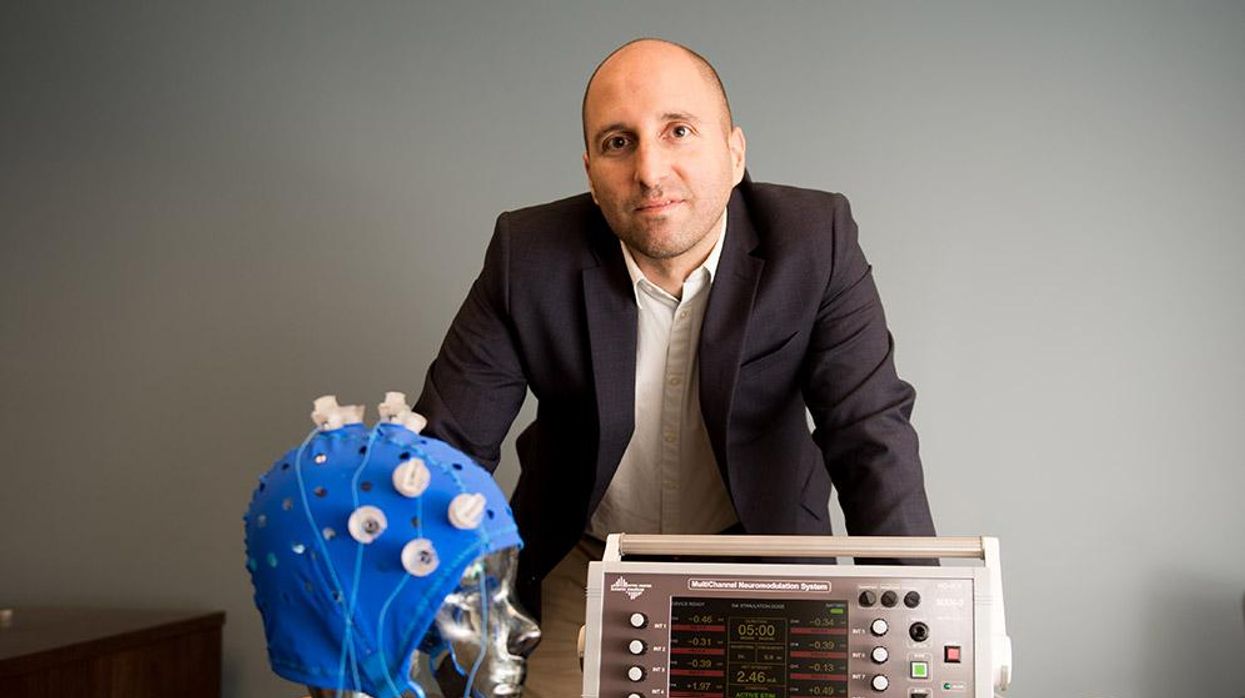
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”