Science Has Given Us the Power to Undermine Nature's Deadliest Creature: Should We Use It?

The Aedes aegypti mosquito, which can carry devastating diseases, was recently engineered by a biotech company to have a genetic "kill switch" intended to crash the local population in the Florida Keys.
Lurking among the swaying palm trees, sugary sands and azure waters of the Florida Keys is the most dangerous animal on earth: the mosquito.
While there are thousands of varieties of mosquitoes, only a small percentage of them are responsible for causing disease. One of the leading culprits is Aedes aegypti, which thrives in the warm standing waters of South Florida, Central America and other tropical climes, and carries the viruses that cause yellow fever, dengue, chikungunya and Zika.
Dengue, a leading cause of death in many Asian and Latin American countries, causes bleeding and pain so severe that it's referred to as "breakbone fever." Chikungunya and yellow fever can both be fatal, and Zika, when contracted by a pregnant woman, can infect her fetus and cause devastating birth defects, including a condition called microcephaly. Babies born with this condition have abnormally small heads and lack proper brain development, which leads to profound, lifelong disabilities.
Decades of efforts to eradicate the disease-carrying Aedes aegypti mosquito from the Keys and other tropical locales have had limited impact. Since the advent of pesticides, homes and neighborhoods have been drenched with them, but after each spraying, the mosquito population quickly bounces back, and the pesticides have to be sprayed over and over. But thanks to genetic engineering, new approaches are underway that could possibly prove safer, cheaper and more effective than any pesticide.
One of those approaches involves, ironically, releasing more mosquitoes in the Florida Keys.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce.
British biotech company Oxitec has engineered male mosquitoes to have a genetic "kill-switch" that could potentially crash the local population of Aedes aegypti, at least in the short-term. The modified males that are being released are intended to mate with wild females.
Males don't bite; it's the female that's deadly, always seeking out blood to gorge on to help mature her eggs. After settling her filament-thin legs on her prey, she sinks a needlelike proboscis into the skin and sucks the blood until her translucent belly is bloated and glowing red.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce. In some experiments using genetically modified mosquitoes, the small number of females that survived were rendered unable to bite. The modification prevented the proboscis, the sickle-like needle that pierces the skin, from forming properly. But this isn't the case with Oxitec's mosquitoes; in the Oxitec release, the females simply die off before they can mate.
The modified mosquitoes are the second genetically engineered insect to be released in the U.S. by Oxitec. The first was a modified diamondback moth, an agricultural pest that doesn't bite humans. But with the mosquitoes, there are many questions about the long-term effects on wild ecosystems, other species in the food chain, and human health. With the Keys initiative, there has been vociferous opposition from environmental groups and some local residents, but some scientists and public health experts say that genetically modified insects pose less of a risk than the diseases they carry and the powerful, indiscriminant pesticides used to combat them.
Oxitec spent a decade developing the technology and engaging in a massive public education campaign before beginning the field test in April. Eventually, the company will release 750,000 of the insects from six locations on three islands of the Florida Keys. Although the release has been approved by the Environmental Protection Agency, the Florida Department of Agriculture and Consumer Services, and the Florida Keys Mosquito Control District, the company was never able to obtain unanimous approval among local residents, some of whom worry that the experiment could cause irreversible damage to the ecosystem.
The company has already begun distributing multiple blue and white boxes containing the eggs of thousands of the mosquitoes which, when water is added, will hatch legions of modified males.
There are a number of techniques available to genetically engineer animals and plants to minimize disease and maximize crop yields. According to Kevin Gorman, chief development officer for Oxitec, the company's mosquitoes were altered by injecting genetic material into the eggs, testing them, then re-injecting them if not enough of the new genes were incorporated into the developing embryos. "We insert genes, but take nothing away," he says.
Gorman points out that the Oxitec mosquitoes will only pass the kill-switch genes on to some of their offspring, and that they will die out fairly quickly. They should temporarily lessen diseases by reducing the local population of Aedes aegypti, but to have a long-term effect, repeated introductions of the altered mosquitoes would have to take place.
Critics say the Oxitec experiment is a precursor to a far more consequential, and more troubling development: the introduction of gene drives in modified species that aggressively tilt inheritance factors in a decided direction.
Gene Drives
Gene drives coupled with the recent development of the gene-editing technique, CRISPR-Cas9, promise to be far more targeted and powerful than previous gene altering efforts. Gene drives override the normal laws of inheritance by harnessing natural processes involved in reproduction. The technique targets small sections of the animal's DNA and replaces it with an altered allele, or trait-determining snippet. Normally, when two members of a species mate, the offspring have a 50 percent chance of receiving an allele because they will receive one from each parent. But in a gene drive, each offspring ends up getting two copies of a desired allele from a single parent—the modified parent. The method "drives" the modified DNA into up to 100 percent of the animals' offspring.
In the case of gene drive mosquitoes, the modified males will mate with wild females. Upon fertilization of the egg, the offspring will start off with one copy of the targeted allele from each parent. But an enzyme, called Cas9, is introduced and acts as a kind of molecular scissors to cut, or damage, the "wild" allele. Then the developing embryo's genetic repair mechanisms kick in and, to repair the damage, copy the undamaged allele from the modified parent. In this way, the offspring ends up with two copies of the modified allele, and it will pass the modification on to virtually all of its progeny.
There is some debate among researchers and others about what constitutes a gene drive, but leaders in the nascent field, such as Andrea Crisanti, generally agree that the defining factor is the heritability of a change introduced into a species. A gene drive is not a particular gene or suite of genes, but a program that proliferates in a species because it is inherited by virtually all offspring.
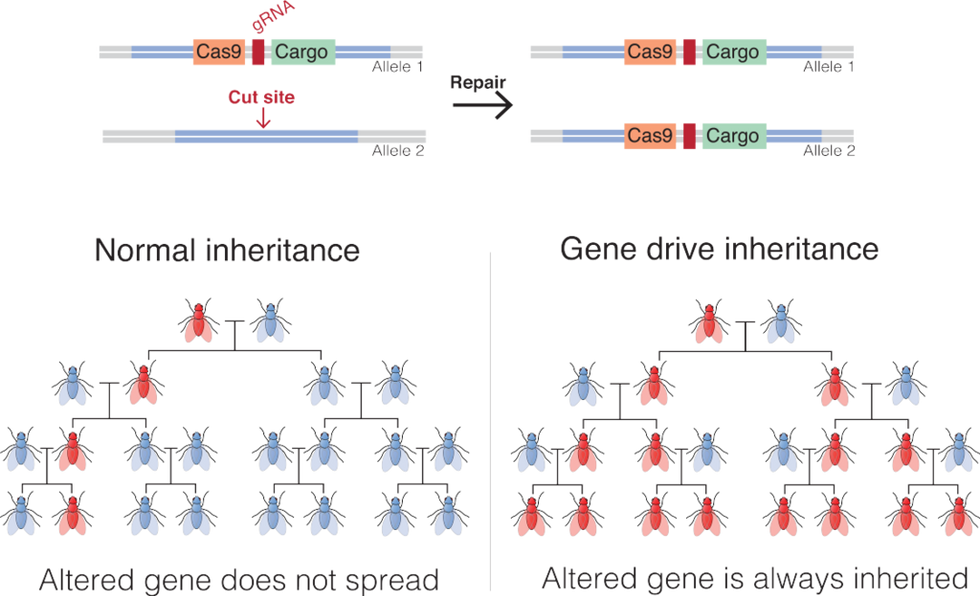
An illustration of how gene drives spread an altered gene through a population.
Mariuswalter, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Of the experts who spoke with Leaps.org for this article, there was disagreement on whether the Oxitec mosquitoes carry a gene drive, but Gorman says they don't because they carry no inheritance advantage. The mosquitoes have baked-in limitations on their potential impact on the tropical ecosystem because the kill-switch should only temporarily affect the local population of Aedes aegypti. The modified mosquitoes will die pretty quickly. But modified organisms that do carry gene drives have the potential to spread widely and persist for an unknown period of time.
Since it has such a reproductive advantage, animals modified by CRISPR and carrying gene drives can quickly replace wild species that compete with them. On the other hand, if the gene drive carries a kill-switch, it can theoretically cause a whole species to collapse.
This makes many people uneasy in an age of mass extinctions, when animals and ecosystems are already under extreme stress due to climate change and the ceaseless destruction of their habitats. Ecosystems are intricate, delicately balanced mosaics where one animal's competitor is another animal's food. The interconnectedness of nature is only partially understood and still contains many mysteries as to what effects human intervention could eventually cause.
But there's a compelling case to be made for the use of gene drives in general. Economies throughout the world are often based on the ecosystem and its animals, which rely on a natural food chain that was evolved over billions of years. But diseases carried by mosquitoes and other animals cause massive damage, both economically and in terms of human suffering.
Malaria alone is a case in point. In 2019, the World Health Organization reported 229 million cases of malaria, which led to 449,000 deaths worldwide. Over 70 percent of those deaths were in children under the age of 12. Efforts to combat malaria-carrying mosquitoes rely on fogging the home with chemical pesticides and sleeping under pesticide-soaked nets, and while this has reduced the occurrence of malaria in recent years, the result is nowhere near as effective as eradicating the Anopheles gambiae mosquito that carries the disease.
Pesticides, a known carcinogen for animals and humans, are a blunt instrument, says Anthony Shelton, a biologist and entomologist at Cornell University. "There are no pesticides so specific that they just get the animal you want to target. They get pollinators. They get predators and parasites. They negatively affect the ecosystem, and they get into our bodies." And it's not uncommon for insects to develop resistance to pesticides, necessitating the continuous development of new, more powerful chemicals to control them.
"The harm of insecticides is not debatable," says Shelton. With gene drives, the potential harm is less clear.
Shelton also points out that although genetic modification sounds radical, people have been altering the genes of animals since before recorded history, through the selective breeding of farm and domesticated animals. While critics of genetic modification decry the possibility of changing the trajectory of evolution in animals, "We've been doing it for centuries," says Shelton. "Gene drives are just a much faster way to do what we've been doing all along."
Still, one might argue that farms are closed experiments, because animals enclosed within farms don't mate with wild animals. This limits the impact of human changes on the larger ecosystem. And getting new genes to work their way through multiple generations in longer-lived animals through breeding can take centuries, which imposes the element of time to ascertain the relative benefits of any introduced change. Gene drives fast-forward change in ways that have never been harnessed before.
The unique thing about gene drives, Shelton says, is that they only affect the targeted species, because those animals will only breed with their own species. Although the Oxitec mosquitoes are modified but not imbued with a gene drive, they illustrate the point. Aedes aegypti will only mate with its own species, and not with any of the other 3,000 varieties of mosquito. According to Shelton, "If they were to disappear, it would have no effect on the fish, bats and birds that feed on them." But should gene drives become widely used, this won't always be true of animals that play a larger part in the food chain. This will be especially true if gene drives are used in mammals.
One factor, cited by both proponents of gene drives and those who want a complete moratorium on them, is that once a gene drive is released into the wild, animals tend to evolve strategies to resist them. In a 2017 article in Nature, Philip Messer, a population geneticist at Cornell, says that gene drives create "the ideal conditions for resistant organisms to flourish."
Sometimes, when CRISPR is used and the Cas9 enzyme cuts an allele soon after egg fertilization, the animal's repair mechanism, rather than creating a straight copy of the desired allele, inserts random DNA letters. The gene drive won't recognize the new sequence, and the change will slip through. In this way, nature has a way of overriding gene drives.
In caged experiments using CRISPR-modified mosquitoes, while the gene drive initially worked, resistance has developed fairly rapidly. Scientists working for Target Malaria, the massive anti-malaria enterprise funded by the Bill and Melinda Gates Foundation, are now working on developing a new version of a gene drive that is not so vulnerable to genetic resistance. But cage conditions are not representative of complex natural ecosystems, and to figure out how a modified species is going to affect the big picture, ultimately they will have to be tested in the wild.
Because there are so many unknowns, such testing is just too dangerous to undertake, according to environmentalists such as Dana Perls of the Friends of the Earth, an international consortium of environmental organizations headquartered in Amsterdam. "There's no safe way to experiment in the wild," she says. "Extinction is permanent, and to drive any species to extinction could have major environmental problems. At a time when we're seeing species disappearing at a high rate, we need to focus on safe processes and a slow approach rather than assume there's a silver bullet."
She cites a number of possible harmful outcomes from genetic modification, including the possible creation of dangerous hybrids that could be more effective at spreading disease and more resistant to pesticides. She points to a 2019 paper in Scientific Reports in which Yale researchers suggested there's evidence that genetically modified species can interbreed with organisms outside their own species. The researchers claimed that when Oxitec tested its modified Aedes aegypti mosquitoes in Brazil, the release resulted in a dangerous hybrid due to the altered animals breeding with two other varieties of mosquito. They suggested that the hybrid mosquito was more robust than the original gene drive mosquitoes.
The paper contributed to breathless headlines in the media and made a big splash with the anti-GMO community. However, it turned out that when other scientists reviewed the data, they found it didn't support the authors' claims. In a short time, the editors of Nature ran an Editorial Expression of Concern for the article, noting that of the insects examined by the researchers, none of them contained the transgenes of the released mosquitoes. Among multiple concerns, Nature found that the researchers didn't follow the released population for more than a short time, and that previous work from the same authors had shown that after a short time, transgenes would have faded from the population.
Of course, unintended consequences are always a concern any time we interfere with nature, says Michael Montague, a senior scholar at Johns Hopkins University's Center for Health Security. "Unpredictability is part of living in the world," he says. Still, he's relatively comfortable with the limited Florida Keys release.
"Even if one type of mosquito was eliminated in the Keys, the ecosystem wouldn't notice," he says. This is because of the thousands of other species of mosquito. He says that while the Keys initiative is ultimately a test, "Oxitec has done their due diligence."
Montague addressed another concern voiced by Perls. The Oxitec mosquitoes were developed so that the female larvae will only hatch in water containing the antibiotic tetracycline. Perls and others caution that, because of the widespread use of antibiotics, the drug inevitably makes its way into the water system, and could be present in the standing pools of water that mosquitoes mate and lay their eggs in.
It's highly unlikely that tetracycline would exist in concentrations high enough to make any difference, says Montague. "But even if it did happen, and the modified females hatched out and mated with wild males, many of their offspring would inherit the modification and only be able to hatch in tetracycline-laced water. The worst-case scenario would be that the pest control didn't work. Net effect: Zero," he says.
As for comparing GMO mosquitoes with insecticides, Montague says, "We 100 percent know insecticides have a harmful effect on human health, whereas modified [male] mosquitoes don't bite humans. They're essentially a chemical-free insecticide, and if there were to be some harmful effect on human health, it would have to be some complicated, convoluted effect" that no one has predicted.
It's not clear, though, given the transitory nature of self-limiting genetically modified insects, whether any effects on the ecosystem would be long-lasting. Certainly in the case of the Oxitec mosquitoes, any effect on the environment would likely be subtle. However, there are other species that are far more important to the food chain, and humans have been greatly impacting them for centuries, sometimes with disastrous effects.
The world's oceans are particularly vulnerable to the effects of human actions. "Codfish used to dominate the North Atlantic ecosystem," says Montague, but due to overfishing, there were huge changes to that ecosystem, including the expansion of their prey—lobsters, crabs and shrimp. The whole system got out of balance." The fish illustrate the international nature of the issues related to gene drives, because wild species have few boundaries and a change in one region can easily spread far and wide.
On the other hand, gene drives can be used for beneficial purposes beyond eliminating disease-carrying species. They could also be used to combat invasive species, fight crop-destroying insects, promote biodiversity, and give a leg up to endangered species that would otherwise die out.
Today nearly 90 percent of the world's islands have been invaded by disease-carrying rodents that have over-multiplied and are driving other island species to extinction. Common rodents such as rats and mice normally encounter a large number of predators in mainland territories, and this controls their numbers. Once they are introduced into island ecosystems, however, they have few predators and often become invasive. Because of this, they are a prevalent cause of the extinction of both animals and plants globally. The primary way to combat them has been to spread powerful toxicants that, when ingested, cause death. Not only has this inhumane practice had limited impact, the toxicants can be eaten by untargeted species and are toxic to humans.
The Genetic Biocontrol of Invasive Rodents program (GBIRd), an international consortium of scientists, ethicists, regulatory experts, sociologists, conservationists and others, is exploring the possible development of a genetically modified mouse that could be introduced to islands where rodents are invasive. Similar to the Oxitec mosquitoes, the mice would carry a modification that results in the appearance of only one sex, and they would also carry a gene drive. Theoretically, once they mate with the wild mice, all of the surviving offspring would be either male or female, and the species would disappear from the islands, giving other, threatened species an opportunity to revive.
GBIRd is moving slowly by design and is currently focused on asking if a genetically engineered mouse should be developed. The program is a potential model for how gene drives can be ethically developed with maximum foresight and the least impact on complex ecosystems. By first releasing a genetically engineered mouse on an island — likely years from now — the impact would naturally be contained within a limited locale.
Regulating GM Insects
While multiple agencies in the U.S. were involved in approving the release of the Oxitec mosquitoes, most experts agree that there is not a straightforward path to regulating genetically modified organisms released into the environment. Clearly, international regulation is needed as genetically modified organisms are released into open environments like the air and the ocean.
The United Nations' Convention on Biological Diversity, which oversees environmental issues at an international level, recently met to continue a process of hammering out voluntary protocols concerning gene drives. Multiple nations have already signed on to already-established protocols, but the United States has not and, according to Montague, is not expected to. "The U.S. will never be signatory to CBD agreements because agricultural companies are huge businesses" that may not see them as in their best interests, he says. Bans or limitations on the release of genetically modified organisms could limit crop yields, for example, thereby limiting profits.
Even if every nation signed on to international regulations of gene drives, cooperation is voluntary. The regulations wouldn't prevent bad actors from using the technology in nefarious ways, such as developing gene drives that can be used as weapons, according to Perls. An example would be unleashing a genetically modified invasive insect to destroy the crops of enemy nations. Or the releasing of a swarm of disease-carrying insects. But in this scenario, it would be very hard to limit the genetically modified species to a specific environment, and the bad actors could be unleashing disaster on themselves.
Because of the risks of misuse, scientists disagree on whether to openly share their gene drive research with others. But Montague believes that there should be a universal registry of gene drives, because "one gene drive can mess up another one. Two groups using the same species should know about each other," he says.
Ultimately, the decision of whether and when to release gene drives into nature rests with not one group, but with society as a whole. This includes not only diverse experts and regulatory bodies, but the general public, a group Oxitec spent considerable time and resources interacting with for their Florida Keys project. In the end, they gained approval for the initiative by a majority of Keys residents, but never gained a total consensus.
There's no escaping the fact that the use of gene drives is a nascent field, and even geneticists and regulators are still grapping with the best ways to develop, oversee, regulate, and control them. Much more data is needed to fully ascertain its risks and benefits.
Experts agree that the Oxitec venture isn't likely to have a noticeable effect on the larger ecosystem unless something truly catastrophic goes wrong. But following the GMO mosquitoes over time will give scientists more real-world data about the long-term effects of genetically altered species. If the release doesn't work, nothing about the ecosystem will change and Aedes aegypti will continue to be a menace to human health. But if something goes horribly wrong, it could hinder the field for years, if not forever.
On the other hand, if the Oxitec mosquitoes and other early initiatives achieve their goals of reducing disease, increasing crop yields, and protecting biodiversity, in the words of Anthony Shelton, "Maybe, 25 to 50 years from now, people will wonder what all the fuss was about."
Correction: The original version of this article mistakenly stated that the modified Oxitec mosquitoes would not be able to form a proper proboscis to bite humans. That is true for some modified mosquitoes but not the Oxitec ones, whose female offspring die off before they reach maturity. Additionally, the Oxitec release was not approved by the FDA and CDC, as originally stated. The FDA and CDC withdrew their role and passed the oversight to other regulatory entities.
Massive benefits of AI come with environmental and human costs. Can AI itself be part of the solution?
Generative AI has a large carbon footprint and other drawbacks. But AI can help mitigate its own harms—by plowing through mountains of data on extreme weather and human displacement.
The recent explosion of generative artificial intelligence tools like ChatGPT and Dall-E enabled anyone with internet access to harness AI’s power for enhanced productivity, creativity, and problem-solving. With their ever-improving capabilities and expanding user base, these tools proved useful across disciplines, from the creative to the scientific.
But beneath the technological wonders of human-like conversation and creative expression lies a dirty secret—an alarming environmental and human cost. AI has an immense carbon footprint. Systems like ChatGPT take months to train in high-powered data centers, which demand huge amounts of electricity, much of which is still generated with fossil fuels, as well as water for cooling. “One of the reasons why Open AI needs investments [to the tune of] $10 billion from Microsoft is because they need to pay for all of that computation,” says Kentaro Toyama, a computer scientist at the University of Michigan. There’s also an ecological toll from mining rare minerals required for hardware and infrastructure. This environmental exploitation pollutes land, triggers natural disasters and causes large-scale human displacement. Finally, for data labeling needed to train and correct AI algorithms, the Big Data industry employs cheap and exploitative labor, often from the Global South.
Generative AI tools are based on large language models (LLMs), with most well-known being various versions of GPT. LLMs can perform natural language processing, including translating, summarizing and answering questions. They use artificial neural networks, called deep learning or machine learning. Inspired by the human brain, neural networks are made of millions of artificial neurons. “The basic principles of neural networks were known even in the 1950s and 1960s,” Toyama says, “but it’s only now, with the tremendous amount of compute power that we have, as well as huge amounts of data, that it’s become possible to train generative AI models.”
Though there aren’t any official figures about the power consumption or emissions from data centers, experts estimate that they use one percent of global electricity—more than entire countries.
In recent months, much attention has gone to the transformative benefits of these technologies. But it’s important to consider that these remarkable advances may come at a price.
AI’s carbon footprint
In their latest annual report, 2023 Landscape: Confronting Tech Power, the AI Now Institute, an independent policy research entity focusing on the concentration of power in the tech industry, says: “The constant push for scale in artificial intelligence has led Big Tech firms to develop hugely energy-intensive computational models that optimize for ‘accuracy’—through increasingly large datasets and computationally intensive model training—over more efficient and sustainable alternatives.”
Though there aren’t any official figures about the power consumption or emissions from data centers, experts estimate that they use one percent of global electricity—more than entire countries. In 2019, Emma Strubell, then a graduate researcher at the University of Massachusetts Amherst, estimated that training a single LLM resulted in over 280,000 kg in CO2 emissions—an equivalent of driving almost 1.2 million km in a gas-powered car. A couple of years later, David Patterson, a computer scientist from the University of California Berkeley, and colleagues, estimated GPT-3’s carbon footprint at over 550,000 kg of CO2 In 2022, the tech company Hugging Face, estimated the carbon footprint of its own language model, BLOOM, as 25,000 kg in CO2 emissions. (BLOOM’s footprint is lower because Hugging Face uses renewable energy, but it doubled when other life-cycle processes like hardware manufacturing and use were added.)
Luckily, despite the growing size and numbers of data centers, their increasing energy demands and emissions have not kept pace proportionately—thanks to renewable energy sources and energy-efficient hardware.
But emissions don’t tell the full story.
AI’s hidden human cost
“If historical colonialism annexed territories, their resources, and the bodies that worked on them, data colonialism’s power grab is both simpler and deeper: the capture and control of human life itself through appropriating the data that can be extracted from it for profit.” So write Nick Couldry and Ulises Mejias, authors of the book The Costs of Connection.
The energy requirements, hardware manufacture and the cheap human labor behind AI systems disproportionately affect marginalized communities.
Technologies we use daily inexorably gather our data. “Human experience, potentially every layer and aspect of it, is becoming the target of profitable extraction,” Couldry and Meijas say. This feeds data capitalism, the economic model built on the extraction and commodification of data. While we are being dispossessed of our data, Big Tech commodifies it for their own benefit. This results in consolidation of power structures that reinforce existing race, gender, class and other inequalities.
“The political economy around tech and tech companies, and the development in advances in AI contribute to massive displacement and pollution, and significantly changes the built environment,” says technologist and activist Yeshi Milner, who founded Data For Black Lives (D4BL) to create measurable change in Black people’s lives using data. The energy requirements, hardware manufacture and the cheap human labor behind AI systems disproportionately affect marginalized communities.
AI’s recent explosive growth spiked the demand for manual, behind-the-scenes tasks, creating an industry described by Mary Gray and Siddharth Suri as “ghost work” in their book. This invisible human workforce that lies behind the “magic” of AI, is overworked and underpaid, and very often based in the Global South. For example, workers in Kenya who made less than $2 an hour, were the behind the mechanism that trained ChatGPT to properly talk about violence, hate speech and sexual abuse. And, according to an article in Analytics India Magazine, in some cases these workers may not have been paid at all, a case for wage theft. An exposé by the Washington Post describes “digital sweatshops” in the Philippines, where thousands of workers experience low wages, delays in payment, and wage theft by Remotasks, a platform owned by Scale AI, a $7 billion dollar American startup. Rights groups and labor researchers have flagged Scale AI as one company that flouts basic labor standards for workers abroad.
It is possible to draw a parallel with chattel slavery—the most significant economic event that continues to shape the modern world—to see the business structures that allow for the massive exploitation of people, Milner says. Back then, people got chocolate, sugar, cotton; today, they get generative AI tools. “What’s invisible through distance—because [tech companies] also control what we see—is the massive exploitation,” Milner says.
“At Data for Black Lives, we are less concerned with whether AI will become human…[W]e’re more concerned with the growing power of AI to decide who’s human and who’s not,” Milner says. As a decision-making force, AI becomes a “justifying factor for policies, practices, rules that not just reinforce, but are currently turning the clock back generations years on people’s civil and human rights.”
Ironically, AI plays an important role in mitigating its own harms—by plowing through mountains of data about weather changes, extreme weather events and human displacement.
Nuria Oliver, a computer scientist, and co-founder and vice-president of the European Laboratory of Learning and Intelligent Systems (ELLIS), says that instead of focusing on the hypothetical existential risks of today’s AI, we should talk about its real, tangible risks.
“Because AI is a transverse discipline that you can apply to any field [from education, journalism, medicine, to transportation and energy], it has a transformative power…and an exponential impact,” she says.
AI's accountability
“At the core of what we were arguing about data capitalism [is] a call to action to abolish Big Data,” says Milner. “Not to abolish data itself, but the power structures that concentrate [its] power in the hands of very few actors.”
A comprehensive AI Act currently negotiated in the European Parliament aims to rein Big Tech in. It plans to introduce a rating of AI tools based on the harms caused to humans, while being as technology-neutral as possible. That sets standards for safe, transparent, traceable, non-discriminatory, and environmentally friendly AI systems, overseen by people, not automation. The regulations also ask for transparency in the content used to train generative AIs, particularly with copyrighted data, and also disclosing that the content is AI-generated. “This European regulation is setting the example for other regions and countries in the world,” Oliver says. But, she adds, such transparencies are hard to achieve.
Google, for example, recently updated its privacy policy to say that anything on the public internet will be used as training data. “Obviously, technology companies have to respond to their economic interests, so their decisions are not necessarily going to be the best for society and for the environment,” Oliver says. “And that’s why we need strong research institutions and civil society institutions to push for actions.” ELLIS also advocates for data centers to be built in locations where the energy can be produced sustainably.
Ironically, AI plays an important role in mitigating its own harms—by plowing through mountains of data about weather changes, extreme weather events and human displacement. “The only way to make sense of this data is using machine learning methods,” Oliver says.
Milner believes that the best way to expose AI-caused systemic inequalities is through people's stories. “In these last five years, so much of our work [at D4BL] has been creating new datasets, new data tools, bringing the data to life. To show the harms but also to continue to reclaim it as a tool for social change and for political change.” This change, she adds, will depend on whose hands it is in.
DNA gathered from animal poop helps protect wildlife
Alida de Flamingh and her team are collecting elephant dung. It holds a trove of information about animal health, diet and genetic diversity.
On the savannah near the Botswana-Zimbabwe border, elephants grazed contentedly. Nearby, postdoctoral researcher Alida de Flamingh watched and waited. As the herd moved away, she went into action, collecting samples of elephant dung that she and other wildlife conservationists would study in the months to come. She pulled on gloves, took a swab, and ran it all over the still-warm, round blob of elephant poop.
Sequencing DNA from fecal matter is a safe, non-invasive way to track and ultimately help protect over 42,000 species currently threatened by extinction. Scientists are using this DNA to gain insights into wildlife health, genetic diversity and even the broader environment. Applied to elephants, chimpanzees, toucans and other species, it helps scientists determine the genetic diversity of groups and linkages with other groups. Such analysis can show changes in rates of inbreeding. Populations with greater genetic diversity adapt better to changes and environmental stressors than those with less diversity, thus reducing their risks of extinction, explains de Flamingh, a postdoctoral researcher at the University of Illinois Urbana-Champaign.
Analyzing fecal DNA also reveals information about an animal’s diet and health, and even nearby flora that is eaten. That information gives scientists broader insights into the ecosystem, and the findings are informing conservation initiatives. Examples include restoring or maintaining genetic connections among groups, ensuring access to certain foraging areas or increasing diversity in captive breeding programs.
Approximately 27 percent of mammals and 28 percent of all assessed species are close to dying out. The IUCN Red List of threatened species, simply called the Red List, is the world’s most comprehensive record of animals’ risk of extinction status. The more information scientists gather, the better their chances of reducing those risks. In Africa, populations of vertebrates declined 69 percent between 1970 and 2022, according to the World Wildlife Fund (WWF).
“We put on sterile gloves and use a sterile swab to collect wet mucus and materials from the outside of the dung ball,” says Alida de Flamingh, a postdoctoral researcher at the University of Illinois Urbana-Champaign.
“When people talk about species, they often talk about ecosystems, but they often overlook genetic diversity,” says Christina Hvilsom, senior geneticist at the Copenhagen Zoo. “It’s easy to count (individuals) to assess whether the population size is increasing or decreasing, but diversity isn’t something we can see with our bare eyes. Yet, it’s actually the foundation for the species and populations.” DNA analysis can provide this critical information.
Assessing elephants’ health
“Africa’s elephant populations are facing unprecedented threats,” says de Flamingh, the postdoc, who has studied them since 2009. Challenges include ivory poaching, habitat destruction and smaller, more fragmented habitats that result in smaller mating pools with less genetic diversity. Additionally, de Flamingh studies the microbial communities living on and in elephants – their microbiomes – looking for parasites or dangerous microbes.
Approximately 415,000 elephants inhabit Africa today, but de Flamingh says the number would be four times higher without these challenges. The IUCN Red List reports African savannah elephants are endangered and African forest elephants are critically endangered. Elephants support ecosystem biodiversity by clearing paths that help other species travel. Their very footprints create small puddles that can host smaller organisms such as tadpoles. Elephants are often described as ecosystems’ engineers, so if they disappear, the rest of the ecosystem will suffer too.
There’s a process to collecting elephant feces. “We put on sterile gloves (which we change for each sample) and use a sterile swab to collect wet mucus and materials from the outside of the dung ball,” says de Flamingh. They rub a sample about the size of a U.S. quarter onto a paper card embedded with DNA preservation technology. Each card is air dried and stored in a packet of desiccant to prevent mold growth. This way, samples can be stored at room temperature indefinitely without the DNA degrading.
Earlier methods required collecting dung in bags, which needed either refrigeration or the addition of preservatives, or the riskier alternative of tranquilizing the animals before approaching them to draw blood samples. The ability to collect and sequence the DNA made things much easier and safer.
“Our research provides a way to assess elephant health without having to physically interact with elephants,” de Flamingh emphasizes. “We also keep track of the GPS coordinates of each sample so that we can create a map of the sampling locations,” she adds. That helps researchers correlate elephants’ health with geographic areas and their conditions.
Although de Flamingh works with elephants in the wild, the contributions of zoos in the United States and collaborations in South Africa (notably the late Professor Rudi van Aarde and the Conservation Ecology Research Unit at the University of Pretoria) were key in studying this method to ensure it worked, she points out.
Protecting chimpanzees
Genetic work with chimpanzees began about a decade ago. Hvilsom and her group at the Copenhagen Zoo analyzed DNA from nearly 1,000 fecal samples collected between 2003 and 2018 by a team of international researchers. The goal was to assess the status of the West African subspecies, which is critically endangered after rapid population declines. Of the four subspecies of chimpanzees, the West African subspecies is considered the most at-risk.
In total, the WWF estimates the numbers of chimpanzees inhabiting Africa’s forests and savannah woodlands at between 173,000 and 300,000. Poaching, disease and human-caused changes to their lands are their major risks.
By analyzing genetics obtained from fecal samples, Hvilsom estimated the chimpanzees’ population, ascertained their family relationships and mapped their migration routes.
“One of the threats is mining near the Nimba Mountains in Guinea,” a stronghold for the West African subspecies, Hvilsom says. The Nimba Mountains are a UNESCO World Heritage Site, but they are rich in iron ore, which is used to make the steel that is vital to the Asian construction boom. As she and colleagues wrote in a recent paper, “Many extractive industries are currently developing projects in chimpanzee habitat.”
Analyzing DNA allows researchers to identify individual chimpanzees more accurately than simply observing them, she says. Normally, field researchers would install cameras and manually inspect each picture to determine how many chimpanzees were in an area. But, Hvilsom says, “That’s very tricky. Chimpanzees move a lot and are fast, so it’s difficult to get clear pictures. Often, they find and destroy the cameras. Also, they live in large areas, so you need a lot of cameras.”
By analyzing genetics obtained from fecal samples, Hvilsom estimated the chimpanzees’ population, ascertained their family relationships and mapped their migration routes based upon DNA comparisons with other chimpanzee groups. The mining companies and builders are using this information to locate future roads where they won’t disrupt migration – a more effective solution than trying to build artificial corridors for wildlife.
“The current route cuts off communities of chimpanzees,” Hvilsom elaborates. That effectively prevents young adult chimps from joining other groups when the time comes, eventually reducing the currently-high levels of genetic diversity.
“The mining company helped pay for the genetics work,” Hvilsom says, “as part of its obligation to assess and monitor biodiversity and the effect of the mining in the area.”
Of 50 toucan subspecies, 11 are threatened or near-threatened with extinction because of deforestation and poaching.
Identifying toucan families
Feces aren't the only substance researchers draw DNA samples from. Jeffrey Coleman, a Ph.D. candidate at the University of Texas at Austin relies on blood tests for studying the genetic diversity of toucans---birds species native to Central America and nearby regions. They live in the jungles, where they hop among branches, snip fruit from trees, toss it in the air and catch it with their large beaks. “Toucans are beautiful, charismatic birds that are really important to the ecosystem,” says Coleman.
Of their 50 subspecies, 11 are threatened or near-threatened with extinction because of deforestation and poaching. “When people see these aesthetically pleasing birds, they’re motivated to care about conservation practices,” he points out.
Coleman works with the Dallas World Aquarium and its partner zoos to analyze DNA from blood draws, using it to identify which toucans are related and how closely. His goal is to use science to improve the genetic diversity among toucan offspring.
Specifically, he’s looking at sections of the genome of captive birds in which the nucleotides repeat multiple times, such as AGATAGATAGAT. Called microsatellites, these consecutively-repeating sections can be passed from parents to children, helping scientists identify parent-child and sibling-sibling relationships. “That allows you to make strategic decisions about how to pair (captive) individuals for mating...to avoid inbreeding,” Coleman says.
Jeffrey Coleman is studying the microsatellites inside the toucan genomes.
Courtesy Jeffrey Coleman
The alternative is to use a type of analysis that looks for a single DNA building block – a nucleotide – that differs in a given sequence. Called single nucleotide polymorphisms (SNPs, pronounced “snips”), they are very common and very accurate. Coleman says they are better than microsatellites for some uses. But scientists have already developed a large body of microsatellite data from multiple species, so microsatellites can shed more insights on relations.
Regardless of whether conservation programs use SNPs or microsatellites to guide captive breeding efforts, the goal is to help them build genetically diverse populations that eventually may supplement endangered populations in the wild. “The hope is that the ecosystem will be stable enough and that the populations (once reintroduced into the wild) will be able to survive and thrive,” says Coleman. History knows some good examples of captive breeding success.
The California condor, which had a total population of 27 in 1987, when the last wild birds were captured, is one of them. A captive breeding program boosted their numbers to 561 by the end of 2022. Of those, 347 of those are in the wild, according to the National Park Service.
Conservationists hope that their work on animals’ genetic diversity will help preserve and restore endangered species in captivity and the wild. DNA analysis is crucial to both types of efforts. The ability to apply genome sequencing to wildlife conservation brings a new level of accuracy that helps protect species and gives fresh insights that observation alone can’t provide.
“A lot of species are threatened,” Coleman says. “I hope this research will be a resource people can use to get more information on longer-term genealogies and different populations.”

