Science Has Given Us the Power to Undermine Nature's Deadliest Creature: Should We Use It?

The Aedes aegypti mosquito, which can carry devastating diseases, was recently engineered by a biotech company to have a genetic "kill switch" intended to crash the local population in the Florida Keys.
Lurking among the swaying palm trees, sugary sands and azure waters of the Florida Keys is the most dangerous animal on earth: the mosquito.
While there are thousands of varieties of mosquitoes, only a small percentage of them are responsible for causing disease. One of the leading culprits is Aedes aegypti, which thrives in the warm standing waters of South Florida, Central America and other tropical climes, and carries the viruses that cause yellow fever, dengue, chikungunya and Zika.
Dengue, a leading cause of death in many Asian and Latin American countries, causes bleeding and pain so severe that it's referred to as "breakbone fever." Chikungunya and yellow fever can both be fatal, and Zika, when contracted by a pregnant woman, can infect her fetus and cause devastating birth defects, including a condition called microcephaly. Babies born with this condition have abnormally small heads and lack proper brain development, which leads to profound, lifelong disabilities.
Decades of efforts to eradicate the disease-carrying Aedes aegypti mosquito from the Keys and other tropical locales have had limited impact. Since the advent of pesticides, homes and neighborhoods have been drenched with them, but after each spraying, the mosquito population quickly bounces back, and the pesticides have to be sprayed over and over. But thanks to genetic engineering, new approaches are underway that could possibly prove safer, cheaper and more effective than any pesticide.
One of those approaches involves, ironically, releasing more mosquitoes in the Florida Keys.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce.
British biotech company Oxitec has engineered male mosquitoes to have a genetic "kill-switch" that could potentially crash the local population of Aedes aegypti, at least in the short-term. The modified males that are being released are intended to mate with wild females.
Males don't bite; it's the female that's deadly, always seeking out blood to gorge on to help mature her eggs. After settling her filament-thin legs on her prey, she sinks a needlelike proboscis into the skin and sucks the blood until her translucent belly is bloated and glowing red.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce. In some experiments using genetically modified mosquitoes, the small number of females that survived were rendered unable to bite. The modification prevented the proboscis, the sickle-like needle that pierces the skin, from forming properly. But this isn't the case with Oxitec's mosquitoes; in the Oxitec release, the females simply die off before they can mate.
The modified mosquitoes are the second genetically engineered insect to be released in the U.S. by Oxitec. The first was a modified diamondback moth, an agricultural pest that doesn't bite humans. But with the mosquitoes, there are many questions about the long-term effects on wild ecosystems, other species in the food chain, and human health. With the Keys initiative, there has been vociferous opposition from environmental groups and some local residents, but some scientists and public health experts say that genetically modified insects pose less of a risk than the diseases they carry and the powerful, indiscriminant pesticides used to combat them.
Oxitec spent a decade developing the technology and engaging in a massive public education campaign before beginning the field test in April. Eventually, the company will release 750,000 of the insects from six locations on three islands of the Florida Keys. Although the release has been approved by the Environmental Protection Agency, the Florida Department of Agriculture and Consumer Services, and the Florida Keys Mosquito Control District, the company was never able to obtain unanimous approval among local residents, some of whom worry that the experiment could cause irreversible damage to the ecosystem.
The company has already begun distributing multiple blue and white boxes containing the eggs of thousands of the mosquitoes which, when water is added, will hatch legions of modified males.
There are a number of techniques available to genetically engineer animals and plants to minimize disease and maximize crop yields. According to Kevin Gorman, chief development officer for Oxitec, the company's mosquitoes were altered by injecting genetic material into the eggs, testing them, then re-injecting them if not enough of the new genes were incorporated into the developing embryos. "We insert genes, but take nothing away," he says.
Gorman points out that the Oxitec mosquitoes will only pass the kill-switch genes on to some of their offspring, and that they will die out fairly quickly. They should temporarily lessen diseases by reducing the local population of Aedes aegypti, but to have a long-term effect, repeated introductions of the altered mosquitoes would have to take place.
Critics say the Oxitec experiment is a precursor to a far more consequential, and more troubling development: the introduction of gene drives in modified species that aggressively tilt inheritance factors in a decided direction.
Gene Drives
Gene drives coupled with the recent development of the gene-editing technique, CRISPR-Cas9, promise to be far more targeted and powerful than previous gene altering efforts. Gene drives override the normal laws of inheritance by harnessing natural processes involved in reproduction. The technique targets small sections of the animal's DNA and replaces it with an altered allele, or trait-determining snippet. Normally, when two members of a species mate, the offspring have a 50 percent chance of receiving an allele because they will receive one from each parent. But in a gene drive, each offspring ends up getting two copies of a desired allele from a single parent—the modified parent. The method "drives" the modified DNA into up to 100 percent of the animals' offspring.
In the case of gene drive mosquitoes, the modified males will mate with wild females. Upon fertilization of the egg, the offspring will start off with one copy of the targeted allele from each parent. But an enzyme, called Cas9, is introduced and acts as a kind of molecular scissors to cut, or damage, the "wild" allele. Then the developing embryo's genetic repair mechanisms kick in and, to repair the damage, copy the undamaged allele from the modified parent. In this way, the offspring ends up with two copies of the modified allele, and it will pass the modification on to virtually all of its progeny.
There is some debate among researchers and others about what constitutes a gene drive, but leaders in the nascent field, such as Andrea Crisanti, generally agree that the defining factor is the heritability of a change introduced into a species. A gene drive is not a particular gene or suite of genes, but a program that proliferates in a species because it is inherited by virtually all offspring.
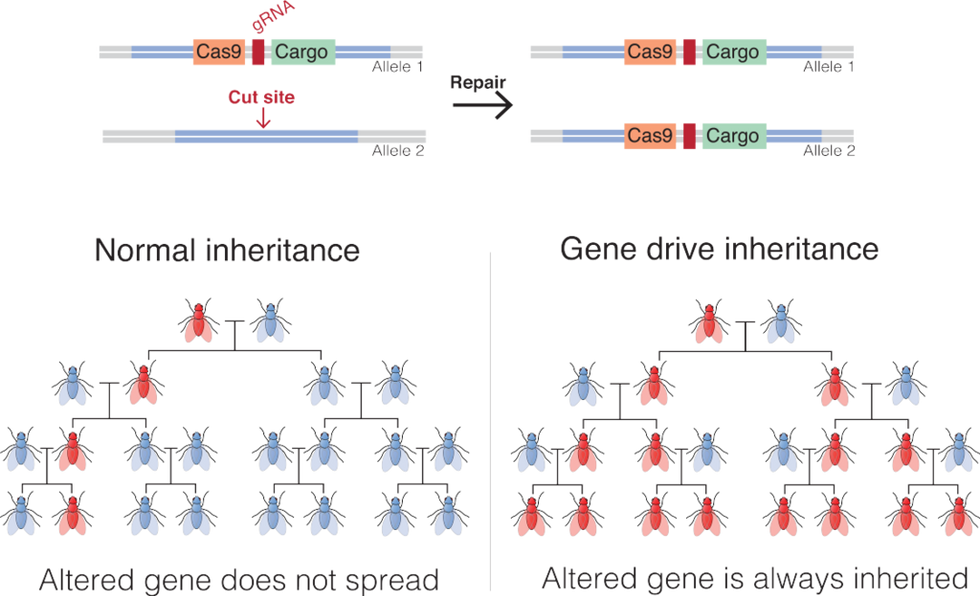
An illustration of how gene drives spread an altered gene through a population.
Mariuswalter, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Of the experts who spoke with Leaps.org for this article, there was disagreement on whether the Oxitec mosquitoes carry a gene drive, but Gorman says they don't because they carry no inheritance advantage. The mosquitoes have baked-in limitations on their potential impact on the tropical ecosystem because the kill-switch should only temporarily affect the local population of Aedes aegypti. The modified mosquitoes will die pretty quickly. But modified organisms that do carry gene drives have the potential to spread widely and persist for an unknown period of time.
Since it has such a reproductive advantage, animals modified by CRISPR and carrying gene drives can quickly replace wild species that compete with them. On the other hand, if the gene drive carries a kill-switch, it can theoretically cause a whole species to collapse.
This makes many people uneasy in an age of mass extinctions, when animals and ecosystems are already under extreme stress due to climate change and the ceaseless destruction of their habitats. Ecosystems are intricate, delicately balanced mosaics where one animal's competitor is another animal's food. The interconnectedness of nature is only partially understood and still contains many mysteries as to what effects human intervention could eventually cause.
But there's a compelling case to be made for the use of gene drives in general. Economies throughout the world are often based on the ecosystem and its animals, which rely on a natural food chain that was evolved over billions of years. But diseases carried by mosquitoes and other animals cause massive damage, both economically and in terms of human suffering.
Malaria alone is a case in point. In 2019, the World Health Organization reported 229 million cases of malaria, which led to 449,000 deaths worldwide. Over 70 percent of those deaths were in children under the age of 12. Efforts to combat malaria-carrying mosquitoes rely on fogging the home with chemical pesticides and sleeping under pesticide-soaked nets, and while this has reduced the occurrence of malaria in recent years, the result is nowhere near as effective as eradicating the Anopheles gambiae mosquito that carries the disease.
Pesticides, a known carcinogen for animals and humans, are a blunt instrument, says Anthony Shelton, a biologist and entomologist at Cornell University. "There are no pesticides so specific that they just get the animal you want to target. They get pollinators. They get predators and parasites. They negatively affect the ecosystem, and they get into our bodies." And it's not uncommon for insects to develop resistance to pesticides, necessitating the continuous development of new, more powerful chemicals to control them.
"The harm of insecticides is not debatable," says Shelton. With gene drives, the potential harm is less clear.
Shelton also points out that although genetic modification sounds radical, people have been altering the genes of animals since before recorded history, through the selective breeding of farm and domesticated animals. While critics of genetic modification decry the possibility of changing the trajectory of evolution in animals, "We've been doing it for centuries," says Shelton. "Gene drives are just a much faster way to do what we've been doing all along."
Still, one might argue that farms are closed experiments, because animals enclosed within farms don't mate with wild animals. This limits the impact of human changes on the larger ecosystem. And getting new genes to work their way through multiple generations in longer-lived animals through breeding can take centuries, which imposes the element of time to ascertain the relative benefits of any introduced change. Gene drives fast-forward change in ways that have never been harnessed before.
The unique thing about gene drives, Shelton says, is that they only affect the targeted species, because those animals will only breed with their own species. Although the Oxitec mosquitoes are modified but not imbued with a gene drive, they illustrate the point. Aedes aegypti will only mate with its own species, and not with any of the other 3,000 varieties of mosquito. According to Shelton, "If they were to disappear, it would have no effect on the fish, bats and birds that feed on them." But should gene drives become widely used, this won't always be true of animals that play a larger part in the food chain. This will be especially true if gene drives are used in mammals.
One factor, cited by both proponents of gene drives and those who want a complete moratorium on them, is that once a gene drive is released into the wild, animals tend to evolve strategies to resist them. In a 2017 article in Nature, Philip Messer, a population geneticist at Cornell, says that gene drives create "the ideal conditions for resistant organisms to flourish."
Sometimes, when CRISPR is used and the Cas9 enzyme cuts an allele soon after egg fertilization, the animal's repair mechanism, rather than creating a straight copy of the desired allele, inserts random DNA letters. The gene drive won't recognize the new sequence, and the change will slip through. In this way, nature has a way of overriding gene drives.
In caged experiments using CRISPR-modified mosquitoes, while the gene drive initially worked, resistance has developed fairly rapidly. Scientists working for Target Malaria, the massive anti-malaria enterprise funded by the Bill and Melinda Gates Foundation, are now working on developing a new version of a gene drive that is not so vulnerable to genetic resistance. But cage conditions are not representative of complex natural ecosystems, and to figure out how a modified species is going to affect the big picture, ultimately they will have to be tested in the wild.
Because there are so many unknowns, such testing is just too dangerous to undertake, according to environmentalists such as Dana Perls of the Friends of the Earth, an international consortium of environmental organizations headquartered in Amsterdam. "There's no safe way to experiment in the wild," she says. "Extinction is permanent, and to drive any species to extinction could have major environmental problems. At a time when we're seeing species disappearing at a high rate, we need to focus on safe processes and a slow approach rather than assume there's a silver bullet."
She cites a number of possible harmful outcomes from genetic modification, including the possible creation of dangerous hybrids that could be more effective at spreading disease and more resistant to pesticides. She points to a 2019 paper in Scientific Reports in which Yale researchers suggested there's evidence that genetically modified species can interbreed with organisms outside their own species. The researchers claimed that when Oxitec tested its modified Aedes aegypti mosquitoes in Brazil, the release resulted in a dangerous hybrid due to the altered animals breeding with two other varieties of mosquito. They suggested that the hybrid mosquito was more robust than the original gene drive mosquitoes.
The paper contributed to breathless headlines in the media and made a big splash with the anti-GMO community. However, it turned out that when other scientists reviewed the data, they found it didn't support the authors' claims. In a short time, the editors of Nature ran an Editorial Expression of Concern for the article, noting that of the insects examined by the researchers, none of them contained the transgenes of the released mosquitoes. Among multiple concerns, Nature found that the researchers didn't follow the released population for more than a short time, and that previous work from the same authors had shown that after a short time, transgenes would have faded from the population.
Of course, unintended consequences are always a concern any time we interfere with nature, says Michael Montague, a senior scholar at Johns Hopkins University's Center for Health Security. "Unpredictability is part of living in the world," he says. Still, he's relatively comfortable with the limited Florida Keys release.
"Even if one type of mosquito was eliminated in the Keys, the ecosystem wouldn't notice," he says. This is because of the thousands of other species of mosquito. He says that while the Keys initiative is ultimately a test, "Oxitec has done their due diligence."
Montague addressed another concern voiced by Perls. The Oxitec mosquitoes were developed so that the female larvae will only hatch in water containing the antibiotic tetracycline. Perls and others caution that, because of the widespread use of antibiotics, the drug inevitably makes its way into the water system, and could be present in the standing pools of water that mosquitoes mate and lay their eggs in.
It's highly unlikely that tetracycline would exist in concentrations high enough to make any difference, says Montague. "But even if it did happen, and the modified females hatched out and mated with wild males, many of their offspring would inherit the modification and only be able to hatch in tetracycline-laced water. The worst-case scenario would be that the pest control didn't work. Net effect: Zero," he says.
As for comparing GMO mosquitoes with insecticides, Montague says, "We 100 percent know insecticides have a harmful effect on human health, whereas modified [male] mosquitoes don't bite humans. They're essentially a chemical-free insecticide, and if there were to be some harmful effect on human health, it would have to be some complicated, convoluted effect" that no one has predicted.
It's not clear, though, given the transitory nature of self-limiting genetically modified insects, whether any effects on the ecosystem would be long-lasting. Certainly in the case of the Oxitec mosquitoes, any effect on the environment would likely be subtle. However, there are other species that are far more important to the food chain, and humans have been greatly impacting them for centuries, sometimes with disastrous effects.
The world's oceans are particularly vulnerable to the effects of human actions. "Codfish used to dominate the North Atlantic ecosystem," says Montague, but due to overfishing, there were huge changes to that ecosystem, including the expansion of their prey—lobsters, crabs and shrimp. The whole system got out of balance." The fish illustrate the international nature of the issues related to gene drives, because wild species have few boundaries and a change in one region can easily spread far and wide.
On the other hand, gene drives can be used for beneficial purposes beyond eliminating disease-carrying species. They could also be used to combat invasive species, fight crop-destroying insects, promote biodiversity, and give a leg up to endangered species that would otherwise die out.
Today nearly 90 percent of the world's islands have been invaded by disease-carrying rodents that have over-multiplied and are driving other island species to extinction. Common rodents such as rats and mice normally encounter a large number of predators in mainland territories, and this controls their numbers. Once they are introduced into island ecosystems, however, they have few predators and often become invasive. Because of this, they are a prevalent cause of the extinction of both animals and plants globally. The primary way to combat them has been to spread powerful toxicants that, when ingested, cause death. Not only has this inhumane practice had limited impact, the toxicants can be eaten by untargeted species and are toxic to humans.
The Genetic Biocontrol of Invasive Rodents program (GBIRd), an international consortium of scientists, ethicists, regulatory experts, sociologists, conservationists and others, is exploring the possible development of a genetically modified mouse that could be introduced to islands where rodents are invasive. Similar to the Oxitec mosquitoes, the mice would carry a modification that results in the appearance of only one sex, and they would also carry a gene drive. Theoretically, once they mate with the wild mice, all of the surviving offspring would be either male or female, and the species would disappear from the islands, giving other, threatened species an opportunity to revive.
GBIRd is moving slowly by design and is currently focused on asking if a genetically engineered mouse should be developed. The program is a potential model for how gene drives can be ethically developed with maximum foresight and the least impact on complex ecosystems. By first releasing a genetically engineered mouse on an island — likely years from now — the impact would naturally be contained within a limited locale.
Regulating GM Insects
While multiple agencies in the U.S. were involved in approving the release of the Oxitec mosquitoes, most experts agree that there is not a straightforward path to regulating genetically modified organisms released into the environment. Clearly, international regulation is needed as genetically modified organisms are released into open environments like the air and the ocean.
The United Nations' Convention on Biological Diversity, which oversees environmental issues at an international level, recently met to continue a process of hammering out voluntary protocols concerning gene drives. Multiple nations have already signed on to already-established protocols, but the United States has not and, according to Montague, is not expected to. "The U.S. will never be signatory to CBD agreements because agricultural companies are huge businesses" that may not see them as in their best interests, he says. Bans or limitations on the release of genetically modified organisms could limit crop yields, for example, thereby limiting profits.
Even if every nation signed on to international regulations of gene drives, cooperation is voluntary. The regulations wouldn't prevent bad actors from using the technology in nefarious ways, such as developing gene drives that can be used as weapons, according to Perls. An example would be unleashing a genetically modified invasive insect to destroy the crops of enemy nations. Or the releasing of a swarm of disease-carrying insects. But in this scenario, it would be very hard to limit the genetically modified species to a specific environment, and the bad actors could be unleashing disaster on themselves.
Because of the risks of misuse, scientists disagree on whether to openly share their gene drive research with others. But Montague believes that there should be a universal registry of gene drives, because "one gene drive can mess up another one. Two groups using the same species should know about each other," he says.
Ultimately, the decision of whether and when to release gene drives into nature rests with not one group, but with society as a whole. This includes not only diverse experts and regulatory bodies, but the general public, a group Oxitec spent considerable time and resources interacting with for their Florida Keys project. In the end, they gained approval for the initiative by a majority of Keys residents, but never gained a total consensus.
There's no escaping the fact that the use of gene drives is a nascent field, and even geneticists and regulators are still grapping with the best ways to develop, oversee, regulate, and control them. Much more data is needed to fully ascertain its risks and benefits.
Experts agree that the Oxitec venture isn't likely to have a noticeable effect on the larger ecosystem unless something truly catastrophic goes wrong. But following the GMO mosquitoes over time will give scientists more real-world data about the long-term effects of genetically altered species. If the release doesn't work, nothing about the ecosystem will change and Aedes aegypti will continue to be a menace to human health. But if something goes horribly wrong, it could hinder the field for years, if not forever.
On the other hand, if the Oxitec mosquitoes and other early initiatives achieve their goals of reducing disease, increasing crop yields, and protecting biodiversity, in the words of Anthony Shelton, "Maybe, 25 to 50 years from now, people will wonder what all the fuss was about."
Correction: The original version of this article mistakenly stated that the modified Oxitec mosquitoes would not be able to form a proper proboscis to bite humans. That is true for some modified mosquitoes but not the Oxitec ones, whose female offspring die off before they reach maturity. Additionally, the Oxitec release was not approved by the FDA and CDC, as originally stated. The FDA and CDC withdrew their role and passed the oversight to other regulatory entities.
New implants let paraplegics surf the web and play computer games
Rodney Gorham, an Australian living with ALS, has reconnected with the world, thanks to a brain-machine interface called the Stentrode.
When I greeted Rodney Gorham, age 63, in an online chat session, he replied within seconds: “My pleasure.”
“Are you moving parts of your body as you type?” I asked.
This time, his response came about five minutes later: “I position the cursor with the eye tracking and select the same with moving my ankles.” Gorham, a former sales representative from Melbourne, Australia, living with amyotrophic lateral sclerosis, or ALS, a rare form of Lou Gehrig’s disease that impairs the brain’s nerve cells and the spinal cord, limiting the ability to move. ALS essentially “locks” a person inside their own body. Gorham is conversing with me by typing with his mind only–no fingers in between his brain and his computer.
The brain-computer interface enabling this feat is called the Stentrode. It's the brainchild of Synchron, a company backed by Amazon’s Jeff Bezos and Microsoft cofounder Bill Gates. After Gorham’s neurologist recommended that he try it, he became one of the first volunteers to have an 8mm stent, laced with small electrodes, implanted into his jugular vein and guided by a surgeon into a blood vessel near the part of his brain that controls movement.
After arriving at their destination, these tiny sensors can detect neural activity. They relay these messages through a small receiver implanted under the skin to a computer, which then translates the information into words. This minimally invasive surgery takes a day and is painless, according to Gorham. Recovery time is typically short, about two days.
When a paralyzed patient thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts.
When a paralyzed patient such as Gorham thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts. This pattern is detected by the Stentrode and relayed to a computer that learns to associate this pattern with the patient’s physical movements. The computer recognizes thoughts about kicking, making a fist and other movements as signals for clicking a mouse or pushing certain letters on a keyboard. An additional eye-tracking device controls the movement of the computer cursor.
The process works on a letter by letter basis. That’s why longer and more nuanced responses often involve some trial and error. “I have been using this for about two years, and I enjoy the sessions,” Gorham typed during our chat session. Zafar Faraz, field clinical engineer at Synchron, sat next to Gorham, providing help when required. Gorham had suffered without internet access, but now he looks forward to surfing the web and playing video games.

Gorham, age 63, has been enjoying Stentrode sessions for about two years.
Rodeny Dekker
The BCI revolution
In the summer of 2021, Synchron became the first company to receive the FDA’s Investigational Device Exemption, which allows research trials on the Stentrode in human patients. This past summer, the company, together with scientists from Icahn School of Medicine at Mount Sinai and the Neurology and Neurosurgery Department at Utrecht University, published a paper offering a framework for how to develop BCIs for patients with severe paralysis – those who can't use their upper limbs to type or use digital devices.
Three months ago, Synchron announced the enrollment of six patients in a study called COMMAND based in the U.S. The company will seek approval next year from the FDA to make the Stentrode available for sale commercially. Meanwhile, other companies are making progress in the field of BCIs. In August, Neuralink announced a $280 million financing round, the biggest fundraiser yet in the field. Last December, Synchron announced a $75 million financing round. “One thing I can promise you, in five years from now, we’re not going to be where we are today. We're going to be in a very different place,” says Elad I. Levy, professor of neurosurgery and radiology at State University of New York in Buffalo.
The risk of hacking exists, always. Cybercriminals, for example, might steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices while extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“The prospect of bestowing individuals with paralysis a renewed avenue for communication and motor functionality is a step forward in neurotech,” says Hayley Nelson, a neuroscientist and founder of The Academy of Cognitive and Behavioral Neuroscience. “It is an exciting breakthrough in a world of devastating, scary diseases,” says Neil McArthur, a professor of philosophy and director of the Centre for Professional and Applied Ethics at the University of Manitoba. “To connect with the world when you are trapped inside your body is incredible.”
While the benefits for the paraplegic community are promising, the Stentrode’s long-term effectiveness and overall impact needs more research on safety. “Potential risks like inflammation, damage to neural tissue, or unexpected shifts in synaptic transmission due to the implant warrant thorough exploration,” Nelson says.
There are also concens about data privacy concerns and the policies of companies to safeguard information processed through BCIs. “Often, Big Tech is ahead of the regulators because the latter didn’t envisage such a turn of events...and companies take advantage of the lack of legal framework to push forward,” McArthur says. Hacking is another risk. Cybercriminals could steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices. Extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“We have to protect patient identity, patient safety and patient integrity,” Levy says. “In the same way that we protect our phones or computers from hackers, we have to stay ahead with anti-hacking software.” Even so, Levy thinks the anticipated benefits for the quadriplegic community outweigh the potential risks. “We are on the precipice of an amazing technology. In the future, we would be able to connect patients to peripheral devices that enhance their quality of life.”
In the near future, the Stentrode could enable patients to use the Stentrode to activate their wheelchairs, iPods or voice modulators. Synchron's focus is on using its BCI to help patients with significant mobility restrictions—not to enhance the lives of healthy people without any illnesses. Levy says we are not prepared for the implications of endowing people with superpowers.
I wondered what Gorham thought about that. “Pardon my question, but do you feel like you have sort of transcended human nature, being the first in a big line of cybernetic people doing marvelous things with their mind only?” was my last question to Gorham.
A slight smile formed on his lips. In less than a minute, he typed: “I do a little.”
Leading XPRIZE Healthspan and Beating Negativity with Dr. Peter Diamandis
XPRIZE founder and chairman Peter Diamandis launches XPRIZE Healthspan at an event on November 29.
A new competition by the XPRIZE Foundation is offering $101 million to researchers who discover therapies that give a boost to people aged 65-80 so their bodies perform more like when they were middle-aged.
For today’s podcast episode, I talked with Dr. Peter Diamandis, XPRIZE’s founder and executive chairman. Under Peter’s leadership, XPRIZE has launched 27 previous competitions with over $300 million in prize purses. The latest contest aims to enhance healthspan, or the period of life when older people can play with their grandkids without any restriction, disability or disease. Such breakthroughs could help prevent chronic diseases that are closely linked to aging. These illnesses are costly to manage and threaten to overwhelm the healthcare system, as the number of Americans over age 65 is rising fast.
In this competition, called XPRIZE Healthspan, multiple awards are available, depending on what’s achieved, with support from the nonprofit Hevolution Foundation and Chip Wilson, the founder of Lululemon and nonprofit SOLVE FSHD. The biggest prize, $81 million, is for improvements in cognition, muscle and immunity by 20 years. An improvement of 15 years will net $71 million, and 10 years will net $61 million.
In our conversation for this episode, Peter talks about his plans for XPRIZE Healthspan and why exponential technologies make the current era - even with all of its challenges - the most exciting time in human history. We discuss the best mental outlook that supports a person in becoming truly innovative, as well as the downsides of too much risk aversion. We talk about how to overcome the negativity bias in ourselves and in mainstream media, how Peter has shifted his own mindset to become more positive over the years, how to inspire a culture of innovation, Peter’s personal recommendations for lifestyle strategies to live longer and healthier, the innovations we can expect in various fields by 2030, the future of education and the importance of democratizing tech and innovation.
In addition to Peter’s pioneering leadership of XPRIZE, he is also the Executive Founder of Singularity University. In 2014, he was named by Fortune as one of the “World’s 50 Greatest Leaders.” As an entrepreneur, he’s started over 25 companies in the areas of health-tech, space, venture capital and education. He’s Co-founder and Vice-Chairman of two public companies, Celularity and Vaxxinity, plus being Co-founder & Chairman of Fountain Life, a fully-integrated platform delivering predictive, preventative, personalized and data-driven health. He also serves as Co-founder of BOLD Capital Partners, a venture fund with a half-billion dollars under management being invested in exponential technologies and longevity companies. Peter is a New York Times Bestselling author of four books, noted during our conversation and in the show notes of this episode. He has degrees in molecular genetics and aerospace engineering from MIT and holds an M.D. from Harvard Medical School.
Show links
- Peter Diamandis bio
- New XPRIZE Healthspan
- Peter Diamandis books
- 27 XPRIZE competitions and counting
- Life Force by Peter Diamandis and Tony Robbins
- Peter Diamandis Twitter
- Longevity Insider newsletter – AI identifies the news
- Peter Diamandis Longevity Handbook
- Hevolution funding for longevity

XPRIZE Founder Peter Diamandis speaks with Mehmoud Khan, CEO of Hevolution Foundation, at the launch of XPRIZE Healthspan.
Hevolution Foundation

