He Beat Lymphoma at 31, While Pioneering Breakthroughs in Cancer Research

Taylor Schreiber, now 39, runs an immunotherapy company testing drugs that may be less toxic alternative to chemotherapy.
It looked like only good things were ahead of Taylor Schreiber in 2010.
Schreiber had just finished his PhD in cancer biology and was preparing to return to medical school to complete his degree. He also had been married a year, and, like any young newlyweds up for adventure, he and his wife Nicki decided to go backpacking in the Costa Rican rainforest.
He was 31, and it was April Fool's Day—but no joke.
During the trip, he experienced a series of night sweats and didn't think too much about it. Schreiber hadn't been feeling right for a few weeks and assumed he had a respiratory infection. Besides, they were sleeping outdoors in a hot, tropical jungle.
But the night sweats continued even after he got home, leaving his mattress so soaked in the morning it was if a bucket of water had been dumped on him overnight. On instinct, he called one of his thesis advisors at the Sylvester Comprehensive Cancer Center in Florida and described his symptoms.
Dr. Joseph Rosenblatt didn't hesitate. "It sounds like Hodgkins. Come see me tomorrow," he said.
The next day, Schreiber was diagnosed with Stage 3b Hodgkin Lymphoma, which meant the disease was advanced. He was 31, and it was April Fool's Day—but no joke.
"I was scared to death," he recalls. "[Thank] goodness it's one of those cancers that is highly treatable. But being 31 years old and all of a sudden being told that you have a 30 percent of mortality within the next two years wasn't anything that I was relieved about."
For Schreiber, the diagnosis was a personal and professional game-changer. He couldn't work in the hospital as a medical student while undergoing chemotherapy, so he wound up remaining in his post-doctorate lab for another two years. The experience also solidified his decision to apply his scientific and medical knowledge to drug development.
Today, now 39, Schreiber is co-founder, director and chief scientific officer of Shattuck Labs, an immuno-oncology startup, and the developer of several important research breakthroughs in the field of immunotherapy.
After his diagnosis, he continued working full-time as a postdoc, while undergoing an aggressive chemotherapy regimen.
"These days, I look back on [my cancer] and think it was one of the luckiest things that ever happened to me," he says. "In medical school, you learn what it is to treat people and learn about the disease. But there is nothing like being a patient to teach you another side of medicine."
Medicine first called to Schreiber when his maternal grandfather was dying from lung cancer complications. Schreiber's uncle, a radiologist at the medical center where his grandfather was being treated, took him on a tour of his department and showed him images of the insides of his body on an ultrasound machine.
Schreiber was mesmerized. His mother was a teacher and his dad sold windows, so medicine was not something to which he had been routinely exposed.
"This weird device was like looking through jelly, and I thought that was the coolest thing ever," he says.
The experience led him to his first real job at the Catholic Medical Center in Manchester, NH, then to a semester-long internship program during his senior year in high school in Concord Hospital's radiology department.
"This was a great experience, but it also made clear that there was not any meaningful way to learn or contribute to medicine before you obtained a medical degree," says Schreiber, who enrolled in Bucknell College to study biology.
Bench science appealed to him, and he volunteered in Dr. Jing Zhou's nephrology department lab at the Harvard Institutes of Medicine. Under the mentorship of one of her post-docs, Lei Guo, he learned a range of critical techniques in molecular biology, leading to their discovery of a new gene related to human polycystic kidney disease and his first published paper.
Before his cancer diagnosis, Schreiber also volunteered in the lab of Dr. Robert "Doc" Sackstein, a world-renowned bone marrow transplant physician and biomedical researcher, and his interests began to shift towards immunology.
"He was just one of those dynamic people who has a real knack for teaching, first of all, and for inspiring people to want to learn more and ask hard questions and understand experimental medicine," Schreiber says.
It was there that he learned the scientific method and the importance of incorporating the right controls in experiments—a simple idea, but difficult to perform well. He also made what Sackstein calls "a startling discovery" about chemokines, which are signaling proteins that can activate an immune response.
As immune cells travel around our bodies looking for potential sources of infection or disease, they latch onto blood vessel walls and "sniff around" for specific chemical cues that indicate a source of infection. Schreiber and his colleagues designed a system that mimics the blood vessel wall, allowing them to define which chemical cues efficiently drive immune cell migration from the blood into tissues.
Schreiber received the best overall research award in 2008 from the National Student Research Foundation. But even as Schreiber's expertise about immunology grew, his own immune system was about to fight its hardest battle.
After his diagnosis, he continued working full-time as a postdoc in the lab of Eckhard Podack, then chair of the microbiology and immunology department at the University of Miami's Leonard M. Miller School of Medicine.
At the same time, Schreiber began an aggressive intravenous chemotherapy regimen of adriamycin, bleomycin, vincristine and dacarbazine, every two weeks, for 6 months. His wife Nicki, an obgyn, transferred her residency from Emory University in Atlanta to Miami so they could be together.
"It was a weird period. I mean, it made me feel good to keep doing things and not just lay idle," he said. "But by the second cycle of chemo, I was immunosuppressed and losing my hair and wore a face mask walking around the lab, which I was certainly self-conscious. But everyone around me didn't make me feel like an alien so I just went about my business."
The experience reinforced his desire to stay in immunology, especially after having taken the most toxic chemotherapies.
He stayed home the day after chemo when he felt his worst, then rested his body and timed exercise to give the drugs the best shot of targeting sick cells (a strategy, he says, that "could have been voodoo"). He also drank "an incredible" amount of fluids to help flush the toxins out of his system.
Side effects of the chemo, besides hair loss, included intense nausea, diarrhea, a loss of appetite, some severe lung toxicities that eventually resolved, and incredible fatigue.
"I've always been a runner, and I would even try to run while I was doing chemo," he said. "After I finished treatment, I would go literally 150 yards and just have to stop, and it took a lot of effort to work through it."
The experience reinforced his desire to stay in immunology, especially after having taken the most toxic chemotherapies.
"They worked, and I could tolerate them because I was young, but people who are older can't," Schreiber said. "The whole field of immunotherapy has really demonstrated that there are effective therapies out there that don't come with all of the same toxicities as the original chemo, so it was galvanizing to imagine contributing to finding some of those."
Schreiber went on to complete his MD and PhD degrees from the Sheila and David Fuente Program in Cancer Biology at the Miller School of Medicine and was nominated in 2011 as a Future Leader in Cancer Research by the American Association for Cancer Research. He also has numerous publications in the fields of tumor immunology and immunotherapy.
Sackstein, who was struck by Schreiber's enthusiasm and "boundless energy," predicts he will be a "major player in the world of therapeutics."
"The future for Taylor is amazing because he has the capacity to synthesize current knowledge and understand the gaps and then ask the right questions to establish new paradigms," said Sackstein, currently dean of the Herbert Wertheim College of Medicine at Florida International University. "It's a very unusual talent."
Since then, he has devoted his career to developing innovative techniques aimed at unleashing the immune system to attack cancer with less toxicity than chemotherapy and better clinical results—first, at a company called Heat Biologics and then at Pelican Therapeutics.
His primary work at Austin, Texas-based Shattuck is aimed at combining two functions in a single therapy for cancer and inflammatory diseases, blocking molecules that put a brake on the immune system (checkpoint inhibitors) while also stimulating the immune system's cancer-killing T cells.
The company has one drug in clinical testing as part of its Agonist Redirected Checkpoint (ARC) platform, which represents a new class of biological medicine. Two others are expected within the next year, with a pipeline of more than 250 drug candidates spanning cancer, inflammatory, and metabolic diseases.
Nine years after his own cancer diagnosis, Schreiber says it remains a huge part of his life, though his chances of a cancer recurrence today are about the same as his chances of getting newly diagnosed with any other cancer.
"I feel blessed to be in a position to help cancer patients live longer and could not imagine a more fulfilling way to spend my life," he says.
Doctors worry that fungal pathogens may cause the next pandemic.
Bacterial antibiotic resistance has been a concern in the medical field for several years. Now a new, similar threat is arising: drug-resistant fungal infections. The Centers for Disease Control and Prevention considers antifungal and antimicrobial resistance to be among the world’s greatest public health challenges.
One particular type of fungal infection caused by Candida auris is escalating rapidly throughout the world. And to make matters worse, C. auris is becoming increasingly resistant to current antifungal medications, which means that if you develop a C. auris infection, the drugs your doctor prescribes may not work. “We’re effectively out of medicines,” says Thomas Walsh, founding director of the Center for Innovative Therapeutics and Diagnostics, a translational research center dedicated to solving the antimicrobial resistance problem. Walsh spoke about the challenges at a Demy-Colton Virtual Salon, one in a series of interactive discussions among life science thought leaders.
Although C. auris typically doesn’t sicken healthy people, it afflicts immunocompromised hospital patients and may cause severe infections that can lead to sepsis, a life-threatening condition in which the overwhelmed immune system begins to attack the body’s own organs. Between 30 and 60 percent of patients who contract a C. auris infection die from it, according to the CDC. People who are undergoing stem cell transplants, have catheters or have taken antifungal or antibiotic medicines are at highest risk. “We’re coming to a perfect storm of increasing resistance rates, increasing numbers of immunosuppressed patients worldwide and a bug that is adapting to higher temperatures as the climate changes,” says Prabhavathi Fernandes, chair of the National BioDefense Science Board.
Most Candida species aren’t well-adapted to our body temperatures so they aren’t a threat. C. auris, however, thrives at human body temperatures.
Although medical professionals aren’t concerned at this point about C. auris evolving to affect healthy people, they worry that its presence in hospitals can turn routine surgeries into life-threatening calamities. “It’s coming,” says Fernandes. “It’s just a matter of time.”
An emerging global threat
“Fungi are found in the environment,” explains Fernandes, so Candida spores can easily wind up on people’s skin. In hospitals, they can be transferred from contact with healthcare workers or contaminated surfaces. Most Candida species aren’t well-adapted to our body temperatures so they aren’t a threat. C. auris, however, thrives at human body temperatures. It can enter the body during medical treatments that break the skin—and cause an infection. Overall, fungal infections cost some $48 billion in the U.S. each year. And infection rates are increasing because, in an ironic twist, advanced medical therapies are enabling severely ill patients to live longer and, therefore, be exposed to this pathogen.
The first-ever case of a C. auris infection was reported in Japan in 2009, although an analysis of Candida samples dated the earliest strain to a 1996 sample from South Korea. Since then, five separate varieties – called clades, which are similar to strains among bacteria – developed independently in different geographies: South Asia, East Asia, South Africa, South America and, recently, Iran. So far, C. auris infections have been reported in 35 countries.
In the U.S., the first infection was reported in 2016, and the CDC started tracking it nationally two years later. During that time, 5,654 cases have been reported to the CDC, which only tracks U.S. data.
What’s more notable than the number of cases is their rate of increase. In 2016, new cases increased by 175 percent and, on average, they have approximately doubled every year. From 2016 through 2022, the number of infections jumped from 63 to 2,377, a roughly 37-fold increase.
“This reminds me of what we saw with epidemics from 2013 through 2020… with Ebola, Zika and the COVID-19 pandemic,” says Robin Robinson, CEO of Spriovas and founding director of the Biomedical Advanced Research and Development Authority (BARDA), which is part of the U.S. Department of Health and Human Services. These epidemics started with a hockey stick trajectory, Robinson says—a gradual growth leading to a sharp spike, just like the shape of a hockey stick.
Another challenge is that right now medics don’t have rapid diagnostic tests for fungal infections. Currently, patients are often misdiagnosed because C. auris resembles several other easily treated fungi. Or they are diagnosed long after the infection begins and is harder to treat.
The problem is that existing diagnostics tests can only identify C. auris once it reaches the bloodstream. Yet, because this pathogen infects bodily tissues first, it should be possible to catch it much earlier before it becomes life-threatening. “We have to diagnose it before it reaches the bloodstream,” Walsh says.
The most alarming fact is that some Candida infections no longer respond to standard therapeutics.
“We need to focus on rapid diagnostic tests that do not rely on a positive blood culture,” says John Sperzel, president and CEO of T2 Biosystems, a company specializing in diagnostics solutions. Blood cultures typically take two to three days for the concentration of Candida to become large enough to detect. The company’s novel test detects about 90 percent of Candida species within three to five hours—thanks to its ability to spot minute quantities of the pathogen in blood samples instead of waiting for them to incubate and proliferate.
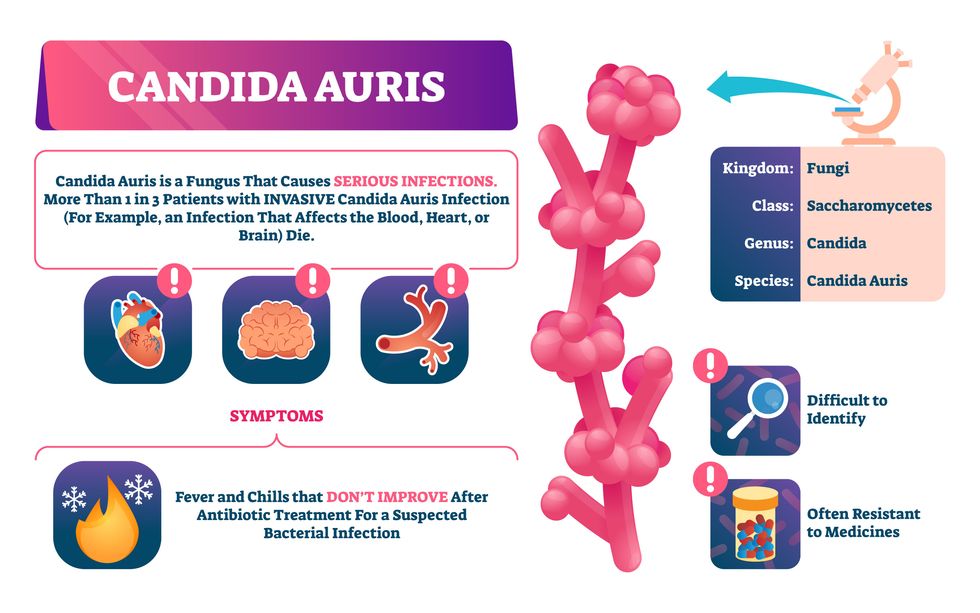
Unlike other Candida species C. auris thrives at human body temperatures
Adobe Stock
Tackling the resistance challenge
The most alarming fact is that some Candida infections no longer respond to standard therapeutics. The number of cases that stopped responding to echinocandin, the first-line therapy for most Candida infections, tripled in 2020, according to a study by the CDC.
Now, each of the first four clades shows varying levels of resistance to all three commonly prescribed classes of antifungal medications, such as azoles, echinocandins, and polyenes. For example, 97 percent of infections from C. auris Clade I are resistant to fluconazole, 54 percent to voriconazole and 30 percent of amphotericin. Nearly half are resistant to multiple antifungal drugs. Even with Clade II fungi, which has the least resistance of all the clades, 11 to 14 percent have become resistant to fluconazole.
Anti-fungal therapies typically target specific chemical compounds present on fungi’s cell membranes, but not on human cells—otherwise the medicine would cause damage to our own tissues. Fluconazole and other azole antifungals target a compound called ergosterol, preventing the fungal cells from replicating. Over the years, however, C. auris evolved to resist it, so existing fungal medications don’t work as well anymore.
A newer class of drugs called echinocandins targets a different part of the fungal cell. “The echinocandins – like caspofungin – inhibit (a part of the fungi) involved in making glucan, which is an essential component of the fungal cell wall and is not found in human cells,” Fernandes says. New antifungal treatments are needed, she adds, but there are only a few magic bullets that will hit just the fungus and not the human cells.
Research to fight infections also has been challenged by a lack of government support. That is changing now that BARDA is requesting proposals to develop novel antifungals. “The scope includes C. auris, as well as antifungals following a radiological/nuclear emergency, says BARDA spokesperson Elleen Kane.
The remaining challenge is the number of patients available to participate in clinical trials. Large numbers are needed, but the available patients are quite sick and often die before trials can be completed. Consequently, few biopharmaceutical companies are developing new treatments for C. auris.
ClinicalTrials.gov reports only two drugs in development for invasive C. auris infections—those than can spread throughout the body rather than localize in one particular area, like throat or vaginal infections: ibrexafungerp by Scynexis, Inc., fosmanogepix, by Pfizer.
Scynexis’ ibrexafungerp appears active against C. auris and other emerging, drug-resistant pathogens. The FDA recently approved it as a therapy for vaginal yeast infections and it is undergoing Phase III clinical trials against invasive candidiasis in an attempt to keep the infection from spreading.
“Ibreafungerp is structurally different from other echinocandins,” Fernandes says, because it targets a different part of the fungus. “We’re lucky it has activity against C. auris.”
Pfizer’s fosmanogepix is in Phase II clinical trials for patients with invasive fungal infections caused by multiple Candida species. Results are showing significantly better survival rates for people taking fosmanogepix.
Although C. auris does pose a serious threat to healthcare worldwide, scientists try to stay optimistic—because they recognized the problem early enough, they might have solutions in place before the perfect storm hits. “There is a bit of hope,” says Robinson. “BARDA has finally been able to fund the development of new antifungal agents and, hopefully, this year we can get several new classes of antifungals into development.”
New elevators could lift up our access to space
A space elevator would be cheaper and cleaner than using rockets
Story by Big Think
When people first started exploring space in the 1960s, it cost upwards of $80,000 (adjusted for inflation) to put a single pound of payload into low-Earth orbit.
A major reason for this high cost was the need to build a new, expensive rocket for every launch. That really started to change when SpaceX began making cheap, reusable rockets, and today, the company is ferrying customer payloads to LEO at a price of just $1,300 per pound.
This is making space accessible to scientists, startups, and tourists who never could have afforded it previously, but the cheapest way to reach orbit might not be a rocket at all — it could be an elevator.
The space elevator
The seeds for a space elevator were first planted by Russian scientist Konstantin Tsiolkovsky in 1895, who, after visiting the 1,000-foot (305 m) Eiffel Tower, published a paper theorizing about the construction of a structure 22,000 miles (35,400 km) high.
This would provide access to geostationary orbit, an altitude where objects appear to remain fixed above Earth’s surface, but Tsiolkovsky conceded that no material could support the weight of such a tower.
We could then send electrically powered “climber” vehicles up and down the tether to deliver payloads to any Earth orbit.
In 1959, soon after Sputnik, Russian engineer Yuri N. Artsutanov proposed a way around this issue: instead of building a space elevator from the ground up, start at the top. More specifically, he suggested placing a satellite in geostationary orbit and dropping a tether from it down to Earth’s equator. As the tether descended, the satellite would ascend. Once attached to Earth’s surface, the tether would be kept taut, thanks to a combination of gravitational and centrifugal forces.
We could then send electrically powered “climber” vehicles up and down the tether to deliver payloads to any Earth orbit. According to physicist Bradley Edwards, who researched the concept for NASA about 20 years ago, it’d cost $10 billion and take 15 years to build a space elevator, but once operational, the cost of sending a payload to any Earth orbit could be as low as $100 per pound.
“Once you reduce the cost to almost a Fed-Ex kind of level, it opens the doors to lots of people, lots of countries, and lots of companies to get involved in space,” Edwards told Space.com in 2005.
In addition to the economic advantages, a space elevator would also be cleaner than using rockets — there’d be no burning of fuel, no harmful greenhouse emissions — and the new transport system wouldn’t contribute to the problem of space junk to the same degree that expendable rockets do.
So, why don’t we have one yet?
Tether troubles
Edwards wrote in his report for NASA that all of the technology needed to build a space elevator already existed except the material needed to build the tether, which needs to be light but also strong enough to withstand all the huge forces acting upon it.
The good news, according to the report, was that the perfect material — ultra-strong, ultra-tiny “nanotubes” of carbon — would be available in just two years.
“[S]teel is not strong enough, neither is Kevlar, carbon fiber, spider silk, or any other material other than carbon nanotubes,” wrote Edwards. “Fortunately for us, carbon nanotube research is extremely hot right now, and it is progressing quickly to commercial production.”Unfortunately, he misjudged how hard it would be to synthesize carbon nanotubes — to date, no one has been able to grow one longer than 21 inches (53 cm).
Further research into the material revealed that it tends to fray under extreme stress, too, meaning even if we could manufacture carbon nanotubes at the lengths needed, they’d be at risk of snapping, not only destroying the space elevator, but threatening lives on Earth.
Looking ahead
Carbon nanotubes might have been the early frontrunner as the tether material for space elevators, but there are other options, including graphene, an essentially two-dimensional form of carbon that is already easier to scale up than nanotubes (though still not easy).
Contrary to Edwards’ report, Johns Hopkins University researchers Sean Sun and Dan Popescu say Kevlar fibers could work — we would just need to constantly repair the tether, the same way the human body constantly repairs its tendons.
“Using sensors and artificially intelligent software, it would be possible to model the whole tether mathematically so as to predict when, where, and how the fibers would break,” the researchers wrote in Aeon in 2018.
“When they did, speedy robotic climbers patrolling up and down the tether would replace them, adjusting the rate of maintenance and repair as needed — mimicking the sensitivity of biological processes,” they continued.Astronomers from the University of Cambridge and Columbia University also think Kevlar could work for a space elevator — if we built it from the moon, rather than Earth.
They call their concept the Spaceline, and the idea is that a tether attached to the moon’s surface could extend toward Earth’s geostationary orbit, held taut by the pull of our planet’s gravity. We could then use rockets to deliver payloads — and potentially people — to solar-powered climber robots positioned at the end of this 200,000+ mile long tether. The bots could then travel up the line to the moon’s surface.
This wouldn’t eliminate the need for rockets to get into Earth’s orbit, but it would be a cheaper way to get to the moon. The forces acting on a lunar space elevator wouldn’t be as strong as one extending from Earth’s surface, either, according to the researchers, opening up more options for tether materials.
“[T]he necessary strength of the material is much lower than an Earth-based elevator — and thus it could be built from fibers that are already mass-produced … and relatively affordable,” they wrote in a paper shared on the preprint server arXiv.
After riding up the Earth-based space elevator, a capsule would fly to a space station attached to the tether of the moon-based one.
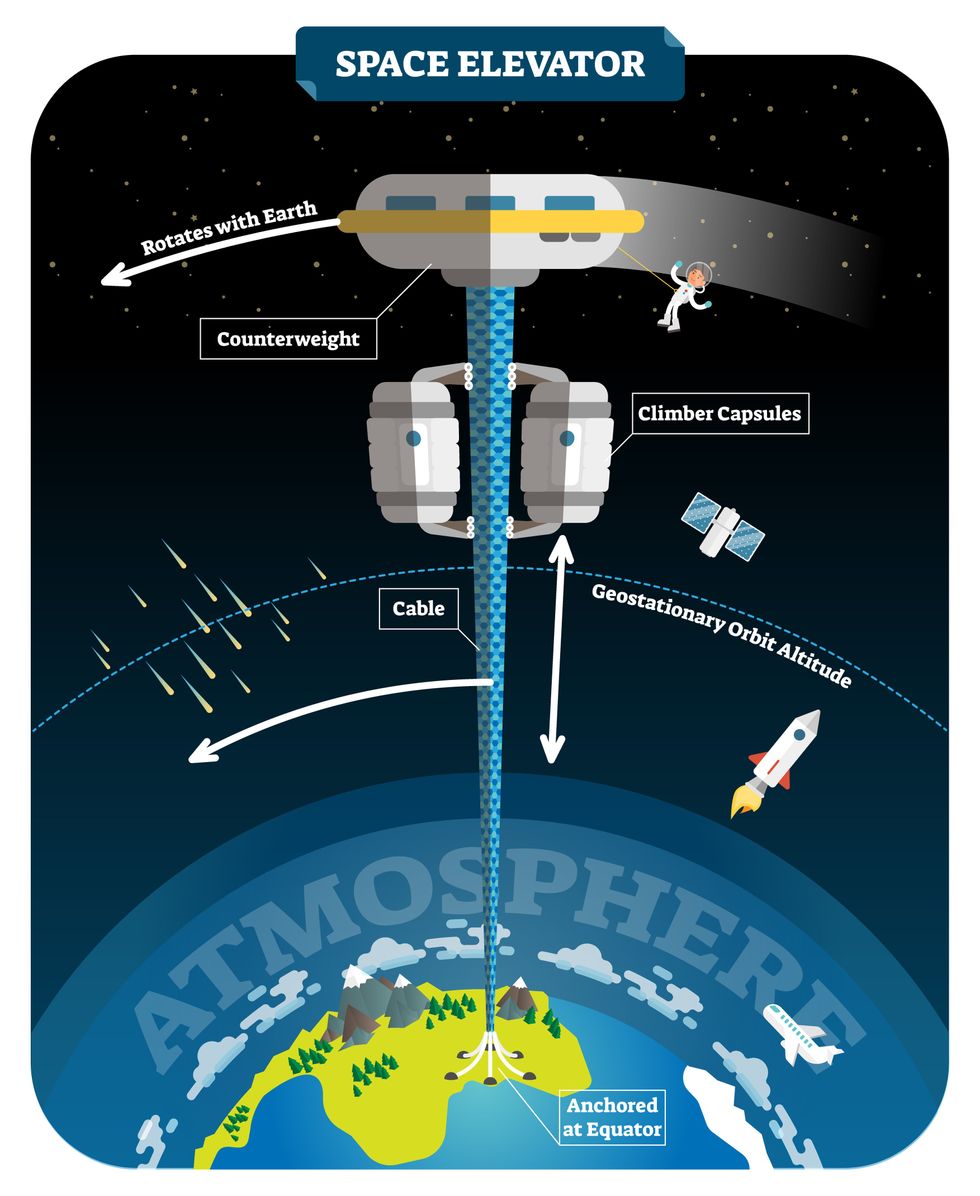
Electrically powered climber capsules could go up down the tether to deliver payloads to any Earth orbit.
Adobe Stock
Some Chinese researchers, meanwhile, aren’t giving up on the idea of using carbon nanotubes for a space elevator — in 2018, a team from Tsinghua University revealed that they’d developed nanotubes that they say are strong enough for a tether.
The researchers are still working on the issue of scaling up production, but in 2021, state-owned news outlet Xinhua released a video depicting an in-development concept, called “Sky Ladder,” that would consist of space elevators above Earth and the moon.
After riding up the Earth-based space elevator, a capsule would fly to a space station attached to the tether of the moon-based one. If the project could be pulled off — a huge if — China predicts Sky Ladder could cut the cost of sending people and goods to the moon by 96 percent.
The bottom line
In the 120 years since Tsiolkovsky looked at the Eiffel Tower and thought way bigger, tremendous progress has been made developing materials with the properties needed for a space elevator. At this point, it seems likely we could one day have a material that can be manufactured at the scale needed for a tether — but by the time that happens, the need for a space elevator may have evaporated.
Several aerospace companies are making progress with their own reusable rockets, and as those join the market with SpaceX, competition could cause launch prices to fall further.
California startup SpinLaunch, meanwhile, is developing a massive centrifuge to fling payloads into space, where much smaller rockets can propel them into orbit. If the company succeeds (another one of those big ifs), it says the system would slash the amount of fuel needed to reach orbit by 70 percent.
Even if SpinLaunch doesn’t get off the ground, several groups are developing environmentally friendly rocket fuels that produce far fewer (or no) harmful emissions. More work is needed to efficiently scale up their production, but overcoming that hurdle will likely be far easier than building a 22,000-mile (35,400-km) elevator to space.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.


