Health breakthroughs of 2022 that should have made bigger news

Nine experts break down the biggest biotech and health breakthroughs that didn't get the attention they deserved in 2022.
As the world has attempted to move on from COVID-19 in 2022, attention has returned to other areas of health and biotech with major regulatory approvals such as the Alzheimer's drug lecanemab – which can slow the destruction of brain cells in the early stages of the disease – being hailed by some as momentous breakthroughs.
This has been a year where psychedelic medicines have gained the attention of mainstream researchers with a groundbreaking clinical trial showing that psilocybin treatment can help relieve some of the symptoms of major depressive disorder. And with messenger RNA (mRNA) technology still very much capturing the imagination, the readouts of cancer vaccine trials have made headlines around the world.
But at the same time there have been vital advances which will likely go on to change medicine, and yet have slipped beneath the radar. I asked nine forward-thinking experts on health and biotech about the most important, but underappreciated, breakthrough of 2022.
Their descriptions, below, were lightly edited by Leaps.org for style and format.
New drug targets for Alzheimer’s disease

Professor Julie Williams, Director, Dementia Research Institute, Cardiff University
Genetics has changed our view of Alzheimer’s disease in the last five to six years. The beta amyloid hypothesis has dominated Alzheimer’s research for a long time, but there are multiple components to this complex disease, of which getting rid of amyloid plaques is one, but it is not the whole story. In April 2022, Nature published a paper which is the culmination of a decade’s worth of work - groups all over the world working together to identify 75 genes associated with risk of developing Alzheimer’s. This provides us with a roadmap for understanding the disease mechanisms.
For example, it is showing that there is something different about the immune systems of people who develop Alzheimer’s disease. There is something different about the way they process lipids in the brain, and very specific processes of how things travel through cells called endocytosis. When it comes to immunity, it indicates that the complement system is affecting whether synapses, which are the connections between neurons, get eliminated or not. In Alzheimer’s this process is more severe, so patients are losing more synapses, and this is correlated with cognition.
The genetics also implicates very specific tissues like microglia, which are the housekeepers in the brain. One of their functions is to clear away beta amyloid, but they also prune and nibble away at parts of the brain that are indicated to be diseased. If you have these risk genes, it seems that you are likely to prune more tissue, which may be part of the cell death and neurodegeneration that we observe in Alzheimer’s patients.
Genetics is telling us that we need to be looking at multiple causes of this complex disease, and we are doing that now. It is showing us that there are a number of different processes which combine to push patients into a disease state which results in the death of connections between nerve cells. These findings around the complement system and other immune-related mechanisms are very interesting as there are already drugs which are available for other diseases which could be repurposed in clinical trials. So it is really a turning point for us in the Alzheimer’s disease field.
Preventing Pandemics with Organ-Tissue Equivalents

Anthony Atala, Director of the Wake Forest Institute for Regenerative Medicine
COVID-19 has shown us that we need to be better prepared ahead of future pandemics and have systems in place where we can quickly catalogue a new virus and have an idea of which treatment agents would work best against it.
At Wake Forest Institute, our scientists have developed what we call organ-tissue equivalents. These are miniature tissues and organs, created using the same regenerative medicine technologies which we have been using to create tissues for patients. For example, if we are making a miniature liver, we will recreate this structure using the six different cell types you find in the liver, in the right proportions, and then the right extracellular matrix which holds the structure together. You're trying to replicate all the characteristics of the liver, but just in a miniature format.
We can now put these organ-tissue equivalents in a chip-like device, where we can expose them to different types of viral infections, and start to get a realistic idea of how the human body reacts to these viruses. We can use artificial intelligence and machine learning to map the pathways of the body’s response. This will allow us to catalogue known viruses far more effectively, and begin storing information on them.
Powering Deep Brain Stimulators with Breath

Islam Mosa, Co-Founder and CTO of VoltXon
Deep brain stimulation (DBS) devices are becoming increasingly common with 150,000 new devices being implanted every year for people with Parkinson’s disease, but also psychiatric conditions such as treatment-resistant depression and obsessive-compulsive disorders. But one of the biggest limitations is the power source – I call DBS devices energy monsters. While cardiac pacemakers use similar technology, their batteries last seven to ten years, but DBS batteries need changing every two to three years. This is because they are generating between 60-180 pulses per second.
Replacing the batteries requires surgery which costs a lot of money, and with every repeat operation comes a risk of infection, plus there is a lot of anxiety on behalf of the patient that the battery is running out.
My colleagues at the University of Connecticut and I, have developed a new way of charging these devices using the person’s own breathing movements, which would mean that the batteries never need to be changed. As the patient breathes in and out, their chest wall presses on a thin electric generator, which converts that movement into static electricity, charging a supercapacitor. This discharges the electricity required to power the DBS device and send the necessary pulses to the brain.
So far it has only been tested in a simulated pig, using a pig lung connected to a pump, but there are plans now to test it in a real animal, and then progress to clinical trials.
Smartwatches for Disease Detection
Jessilyn Dunn, Assistant Professor in Duke Biomedical Engineering
A group of researchers recently showed that digital biomarkers of infection can reveal when someone is sick, often before they feel sick. The team, which included Duke biomedical engineers, used information from smartwatches to detect Covid-19 cases five to 10 days earlier than diagnostic tests. Smartwatch data included aspects of heart rate, sleep quality and physical activity. Based on this data, we developed an algorithm to decide which people have the most need to take the diagnostic tests. With this approach, the percent of tests that come back positive are about four- to six-times higher, depending on which factors we monitor through the watches.
Our study was one of several showing the value of digital biomarkers, rather than a single blockbuster paper. With so many new ideas and technologies coming out around Covid, it’s hard to be that signal through the noise. More studies are needed, but this line of research is important because, rather than treat everyone as equally likely to have an infectious disease, we can use prior knowledge from smartwatches. With monkeypox, for example, you've got many more people who need to be tested than you have tests available. Information from the smartwatches enables you to improve how you allocate those tests.
Smartwatch data could also be applied to chronic diseases. For viruses, we’re looking for information about anomalies – a big change point in people’s health. For chronic diseases, it’s more like a slow, steady change. Our research lays the groundwork for the signals coming from smartwatches to be useful in a health setting, and now it’s up to us to detect more of these chronic cases. We want to go from the idea that we have this single change point, like a heart attack or stroke, and focus on the part before that, to see if we can detect it.
A Vaccine For RSV

Norbert Pardi, Vaccines Group Lead, Penn Institute for RNA Innovation, University of Pennsylvania
Scientists have long been trying to develop a vaccine for respiratory syncytial virus (RSV), and it looks like Pfizer are closing in on this goal, based on the latest clinical trial data in newborns which they released in November. Pfizer have developed a protein-based vaccine against the F protein of RSV, which they are giving to pregnant women. It turns out that it induces a robust immune response after the administration of a single shot and it seems to be highly protective in newborns. The efficacy was over 80% after 90 days, so it protected very well against severe disease, and even though this dropped a little after six month, it was still pretty high.
I think this has been a very important breakthrough, and very timely at the moment with both COVID-19, influenza and RSV circulating, which just shows the importance of having a vaccine which works well in both the very young and the very old.
The road to an RSV vaccine has also illustrated the importance of teamwork in 21st century vaccine development. You need people with different backgrounds to solve these challenges – microbiologists, immunologists and structural biologists working together to understand how viruses work, and how our immune system induces protective responses against certain viruses. It has been this kind of teamwork which has yielded the findings that targeting the prefusion stabilized form of the F protein in RSV induces much stronger and highly protective immune responses.
Gene therapy shows its potential

Nicole Paulk, Assistant Professor of Gene Therapy at the University of California, San Francisco
The recent US Food and Drug Administration (FDA) approval of Hemgenix, a gene therapy for hemophilia B, is big for a lot of reasons. While hemophilia is absolutely a rare disease, it is astronomically more common than the first two approvals – Luxturna for RPE65-meidated inherited retinal dystrophy and Zolgensma for spinal muscular atrophy - so many more patients will be treated with this. In terms of numbers of patients, we are now starting to creep up into things that are much more common, which is a huge step in terms of our ability to scale the production of an adeno-associated virus (AAV) vector for gene therapy.
Hemophilia is also a really special patient population because this has been the darling indication for AAV gene therapy for the last 20 to 30 years. AAV trafficks to the liver so well, it’s really easy for us to target the tissues that we want. If you look at the numbers, there have been more gene therapy scientists working on hemophilia than any other condition. There have just been thousands and thousands of us working on gene therapy indications for the last 20 or 30 years, so to see the first of these approvals make it, feels really special.
I am sure it is even more special for the patients because now they have a choice – do I want to stay on my recombinant factor drug that I need to take every day for the rest of my life, or right now I could get a one-time infusion of this virus and possibly experience curative levels of expression for the rest of my life. And this is just the first one for hemophilia, there’s going to end up being a dozen gene therapies within the next five years, targeted towards different hemophilias.
Every single approval is momentous for the entire field because it gets investors excited, it gets companies and physicians excited, and that helps speed things up. Right now, it's still a challenge to produce enough for double digit patients. But with more interest comes the experiments and trials that allow us to pick up the knowledge to scale things up, so that we can go after bigger diseases like diabetes, congestive heart failure, cancer, all of these much bigger afflictions.
Treating Thickened Hearts

John Spertus, Professor in Metabolic and Vascular Disease Research, UMKC School of Medicine
Hypertrophic cardiomyopathy (HCM) is a disease that causes your heart muscle to enlarge, and the walls of your heart chambers thicken and reduce in size. Because of this, they cannot hold as much blood and may stiffen, causing some sufferers to experience progressive shortness of breath, fatigue and ultimately heart failure.
So far we have only had very crude ways of treating it, using beta blockers, calcium channel blockers or other medications which cause the heart to beat less strongly. This works for some patients but a lot of time it does not, which means you have to consider removing part of the wall of the heart with surgery.
Earlier this year, a trial of a drug called mavacamten, became the first study to show positive results in treating HCM. What is remarkable about mavacamten is that it is directed at trying to block the overly vigorous contractile proteins in the heart, so it is a highly targeted, focused way of addressing the key problem in these patients. The study demonstrated a really large improvement in patient quality of life where they were on the drug, and when they went off the drug, the quality of life went away.
Some specialists are now hypothesizing that it may work for other cardiovascular diseases where the heart either beats too strongly or it does not relax well enough, but just having a treatment for HCM is a really big deal. For years we have not been very aggressive in identifying and treating these patients because there have not been great treatments available, so this could lead to a new era.
Regenerating Organs

David Andrijevic, Associate Research Scientist in neuroscience at Yale School of Medicine
As soon as the heartbeat stops, a whole chain of biochemical processes resulting from ischemia – the lack of blood flow, oxygen and nutrients – begins to destroy the body’s cells and organs. My colleagues and I at Yale School of Medicine have been investigating whether we can recover organs after prolonged ischemia, with the main goal of expanding the organ donor pool.
Earlier this year we published a paper in which we showed that we could use technology to restore blood circulation, other cellular functions and even heart activity in pigs, one hour after their deaths. This was done using a perfusion technology to substitute heart, lung and kidney function, and deliver an experimental cell protective fluid to these organs which aimed to stop cell death and aid in the recovery.
One of the aims of this technology is that it can be used in future to lengthen the time window for recovering organs for donation after a person has been declared dead, a logistical hurdle which would allow us to substantially increase the donor pool. We might also be able to use this cell protective fluid in studies to see if it can help people who have suffered from strokes and myocardial infarction. In future, if we managed to achieve an adequate brain recovery – and the brain, out of all the organs, is the most susceptible to ischemia – this might also change some paradigms in resuscitation medicine.
Antibody-Drug Conjugates for Cancer

Yosi Shamay, Cancer Nanomedicine and Nanoinformatics researcher at the Technion Israel Institute of Technology
For the past four or five years, antibody-drug conjugates (ADCs) - a cancer drug where you have an antibody conjugated to a toxin - have been used only in patients with specific cancers that display high expression of a target protein, for example HER2-positive breast cancer. But in 2022, there have been clinical trials where ADCs have shown remarkable results in patients with low expression of HER2, which is something we never expected to see.
In July 2022, AstraZeneca published the results of a clinical trial, which showed that an ADC called trastuzumab deruxtecan can offer a very big survival benefit to breast cancer patients with very little expression of HER2, levels so low that they would be borderline undetectable for a pathologist. They got a strong survival signal for patients with very aggressive, metastatic disease.
I think this is very interesting and important because it means that it might pave the way to include more patients in clinical trials looking at ADCs for other cancers, for example lymphoma, colon cancer, lung cancers, even if they have low expression of the protein target. It also holds implications for CAR-T cells - where you genetically engineer a T cell to attack the cancer - because the concept is very similar. If we now know that an ADC can have a survival benefit, even in patients with very low target expression, the same might be true for T cells.
Look back further: Breakthroughs of 2021

https://leaps.org/6-biotech-breakthroughs-of-2021-that-missed-the-attention-they-deserved/
How One University Is Successfully Tackling COVID-19
Alma Mater, a beloved bronze mother figure on the campus of the University of Illinois, Champaign-Urbana, wears a mask to encourage students to do the same as they return for the fall semester. Students are also expected to take a COVID-19 test twice a week.
China, South Korea and other places controlled the SARS-CoV-2 epidemic with the early use of strict lockdown and aggressive electronic contact tracing, monitoring, and enforcement.
The tussles in America over voluntary social distancing and wearing a mask in public suggest that more stringent enforcement methods adopted elsewhere would not work here. But one American university has emerged as a model of tough love pandemic management.
While many universities have become hot spots of COVID-19 infections this fall when students returned to campus, the University of Illinois was an exception. It has gotten the virus under control, at least for the moment, at a rate that is far below the national average and with minimal social disruption. Can the program they implemented work in our broader society?
The Illinois model is a comprehensive one which, as elsewhere, includes masking and social distancing, but it also requires a twice-weekly saliva test for SARS-CoV-2. All students and employees are assigned test days when they swipe their ID card and spit in a plastic tube, which is collected hourly and taken to a campus lab.
There a simplified but highly sensitive PCR genetic test goes through many cycles of amplifying the viral RNA. "Tracking three different viral RNA [genes] gives us very high accuracy," explains Martin Burke, the professor who developed the system and is monitoring its implementation at the University of Illinois Urbana-Champaign. They immediately retest any positive sample to confirm the results, "So we think our false positive rate is extremely low. … The goal is to notify the positive person within 30 minutes of a positive test results becoming known."
Testing everyone so frequently, with a sensitive test that can quickly detect small amounts of the virus soon after infection, and isolating those who test positive before the virus can grow to volumes that make it very infectious helps the Illinois system break the chain of transmission.
"The testing we have done is not a silver bullet, it has to be done in combination with other mitigation measures. Our modeling shows that if you have masks, social distancing, and contact tracing you get a very dramatic, in fact synergistic effect with this combination,' says Burke. "So it really has to be a holistic approach with lots of community engagement in order to make this process successful."
The real teeth of enforcement are that people have to display their health status to gain access to campus facilities. A green check mark over their photo on a college ID phone app means they are good to go but a big red X means they are not current on their testing or have tested positive for the virus. Their ID is inactivated and they cannot enter campus facilities until they become compliant. Burke puts it bluntly; "We stop them from going where they want to go, a measure first used successfully with the pandemic in Wuhan, China.
He says they have learned from their experience and evolved their approach. "We never modeled for people who tested positive to ignore that result and go to or host parties, which could spread the infection." But several students did just that, and a few have been suspended for it.
So the university clamped down on enforcing isolation and now requires some higher risk persons to test three times a week to catch any infections earlier. Since more than 95 percent of new infections were among undergraduates, with no crossover from them to the local community, faculty, or graduate students, they have cut back testing of the latter two groups to just once a week.
About a thousand positive tests results have come back so far but no one has been hospitalized. Part of that likely is because the undergraduate population is largely young and healthy with few risk cofactors. But it may also be that with early identification and isolation, about five percent of dorm rooms have been set aside for that purpose, the person adopts healthier patterns of sleeping and eating that allows the immune system to better fight off the virus.
"But when you compare that to the being able to educate our students, perform research, keep our community thriving, our businesses open, if you add it all up, it's a tremendous return on investment."
The logistics are quite impressive for the campus that in ordinary times is home to more than 50,000 students; a lab capable of churning through 20,000 tests a day, with notification of results within hours, not days as is common elsewhere. And the results are equally impressive. The rate of positive test results blipped up to around 3 percent when undergraduates arrived back on campus but that has plummeted to 0.35 percent for the last seven-day period of testing, a tiny fraction of the rate for the nation as a whole. Much of it can be attributed to the closed environment with limited outside contact that might reintroduce the virus.
Still, even while the campus population has dropped by about a third, they are detecting about 250 new infections a week.
The threat of outside contact adding to the risk is why the university amended the undergraduate school calendar to close for Thanksgiving, hold final classes and exams for the semester online, and not return until February.
It doesn't come cheap. Burke estimates it cost $10 million to set up the program and about the same each semester to operate. "But when you compare that to the being able to educate our students, perform research, keep our community thriving, our businesses open, if you add it all up, it's a tremendous return on investment."
Burke acknowledges that they started with some significant advantages. The community is geographically isolated, an electronically linked ID system was already in place for students and employees, they have the ability to control much activity through access to buildings, and they can expel those who do not conform. He believes their system can translate to similar settings but admits, "A big city is very different from a university community." Still, he believes many of those lessons can be translated to different settings.
An alternative story
However, the situation is very different at the University of Colorado, where new infections have surged since undergraduates returned in late August. Administrators recently switched all classes to online only in an attempt to control the virus.
But that wasn't enough for state authorities who cracked down further, just yesterday declaring a two-week lockdown of all students aged 18 to 22, prohibiting gatherings of any size, indoors or out. Students must stay in their rooms except for essential activities, and if any symptoms develop, report for testing. Fraternities and sororities were targeted as past hot spots of infection.
The police will be actively enforcing the lockdown, and violators can face a penalty of up to 90 days in jail and a $1,000 fine.
Skepticism
Public health largely is based upon an appeal to self-interest and altruism, and voluntary compliance with official guidance. Harm reduction often comes into play when an ideal solution meets resistance and coercion plays only a limited role, as when a person with infectious tuberculosis is not compliant with treatment. Many question whether the medical threat of COVID-19 justifies such a sweeping restriction of individual rights of movement and association imposed on everyone simply because of their age and place of residence as is happening in Colorado.
State and federal courts have begun to strike down as an unconstitutional overreach some of the more restrictive decrees to stay at home or close businesses ordered by state and local officials. What was once tolerated as a few weeks or even a few months of restrictions now seems to stretch without an end in sight, and threatens peoples' livelihoods. In this litigious country it seems only a matter of time before someone will challenge some aspects of the Illinois model or similar programs being set up elsewhere as an infringement of their rights.
"I have real concerns about what we have seen over the course of the past several months in terms of going from not enough testing being available to now having more testing [available] because people don't want to be tested, even when they have symptoms," says Michael Osterholm, a noted expert on pandemic preparedness at the University of Minnesota. "We have some college campuses reporting over fifty percent of the students refusing to be tested or refusing to give any of the contacts that might be followed up on."
Often those who have tested positive for the virus "don't want people to know that they're the potential reason there could be an outbreak in their small social circle," says LaQuandra Nesbitt, public health director for Washington, DC. Stigma is one of the main reasons why only 37% of newly infected people have provided names for contact tracing in D.C., and few offer more than a single name.
"We can't test every single person every single day, we would completely go broke, we would be looking at no other health problems. We're not the NFL," says Monica Gandhi. She is a professor of medicine at the University of California San Francisco and works closely with local health officials. "Just because we have a technology doesn't mean that we have to apply it for every purpose that may be indicated. … We would never dream of mass screening the public for influenza."
"Tests don't solve the problem," she argues. Masking is the most crucial piece for Gandhi, along with social distancing, washing hands regularly, and quarantine when testing positive or in contact with someone who is. Those are the actions that break the ongoing spread of transmission. She does support regular testing in high-risk settings such as nursing homes, inpatients in hospitals, and prisons, and periodic surveys in the general population to better understand where the virus is moving.
Drawing from experience with HIV, Gandhi worries that the stigma of a positive result will drive people away from testing. "Low-income persons will be particularly hesitant to get tested, or to share contact information if they do test positive, if they think they may have to quarantine, not work or gain income." That is why San Francisco initially assisted people in isolation with payment of $1285 for two weeks of isolation and other support as part of a right to health program. And this fall, the State of California passed legislation requiring that large businesses continue to pay employees in quarantine.
Tools for self-protection
The American temperament, decentralization, size, administrative complexity, and sheer cost make it highly unlikely that a coercive one-size-fits-all Illinois approach will ever be rolled out from a university campus to the entire nation. People make different decisions in trading off between safety and personal freedom or autonomy, and many are likely to embrace a rapid, inexpensive self-test if one becomes available, much like a home pregnancy test, to proactively monitor their own health.
OraSure Technologies pioneered the first home test for HIV. It is the only over-the-counter saliva test for HIV approved for sale in the U.S. Results show in about 20 minutes. The company went on to develop versions of this test for hepatitis C and Ebola. Thus it came as no surprise when in April the Department of Health and Human Services awarded it a $710 thousand contract to develop a rapid antigen home test for SARS-CoV-2.
Initial optimization studies for the antigen test showed that a nasal sample rather than an oral one generated better results, OraSure president and CEO Stephen Tang told LeapsMag. A test using a nasal swab is expected to be available later this year while work continues to develop an antibody test that uses saliva. He says, "the fundamental challenge is not only to develop the tests but to get it to scale quickly. That's the only way it's really going to matter." The company has manufacturing capacity to produce 35 million tests a year, with about half for SARS-CoV-2, and will double that capacity in steps within the next twelve months, with all of the increased capacity dedicated to COVID-19.
Initial use will be limited to health care workers and by prescription, but the company hopes to make it available over the counter soon after the FDA finalizes its rules on these types of tests for COVID-19. Importantly, OraSure believes its nasal swab test will be able to meet the current FDA standards for at-home tests. No such tests have yet been approved.
Tang says they envision using a phone app with the test, but that's tied to "the question of our century; who owns the data? If you are an individual buying the test, are you really compelled to report to anybody? If you are an employer and you buy the test and your employees take it, are you then entitled to the information because you're the one administering the test? That's all still being debated as well" by regulators, lawyers, and ethicists.
The price hasn't been set but Tang notes that they have "vast experience" in selling directly to the consumer, physicians, and public health systems in the U.S. and in lower-income companies. "We are very aware of what the economics are and what the need is today. We're trying to make this product as widely available to as many people as possible."
Another tool that may help protect the self-motivated are cell phone apps that alert you to potential exposure to others with the virus. Apple, Google and others have developed versions of the app that all work on the same principle and, miraculously, are compatible between the Apple and Android operating system universes. At first glance they look promising.
The glitch is that where they have been available the longest, only about 15-20 percent of users bother to download it, says Bennett Cyphers, a staff technologist with the Electronic Freedom Foundation (EFF), a nonprofit that advocates for privacy and other concerns in cyberspace. He explains, "If 1 in 10 people have the app installed, then only 1 in 100 interactions between everyone is going to be captured by the app. It scales that way; the fewer people you have, then a really, really small fraction of contacts are actually detected."
It is important to remember that much of public health is not the result of policy but of what people do in their daily lives.
Importantly, about 20 percent of Americans do not own a smart phone with the capacity to handle the app; that percentage is even higher among lower income, less educated, older folks who often are most at risk for suffering a severe case of COVID-19. So the value of this tool is likely to remain largely theoretical.
Divining the future
"It's tough to make predictions, especially about the future," the great baseball sage Yogi Berra is reported to have said. Will the COVID-19 pandemic in the U.S. follow the path of Illinois or Colorado?
The recent past often is no guide to such predictions. France, Spain, and Israel once earned plaudits for early and strict enforcement of lockdowns to control spread of the virus and then eased up on those restrictions. At the same time the world watched with condemnation and fascination as Sweden chose to follow a more laissez faire approach, urging voluntary distancing and masking but no major curtailing of activity.
Today the rates of new infections of COVID-19 in the first three countries have exploded to equal or multiples of the rate in Sweden. Which approach was the correct policy? Most people say it is still too early to tell for sure. The same can be said for the examples of Illinois and Colorado.
And then there is the puzzling example of Manaus, the Brazilian city of 1.8 million in the middle of the Amazon which was slammed with infections as hard as New York City; without the medical infrastructure to cope with the virus, 4000 have died. But then, suddenly, new infections began to taper off, and nobody claims to understand why, it certainly wasn't because official policies changed. One guess is that perhaps the region reached herd immunity, but that is simply speculation.
One can pick and choose examples of tough enforcement of quarantine or none to prove their point for the short term. But draconian measures will not be tolerated for long in a free society, and there is no clear, overwhelming evidence that over the long run one policy approach works better than another.
It is important to remember that much of public health is not the result of policy but of what people do in their daily lives. We have come remarkably far in what is still only months since we first heard the name of the virus. Death rates have fallen dramatically as we have learned how to better manage severe disease, often by adapting treatments for other diseases. And there is reason for optimism with the large number of vaccine candidates already in human trials.
We also have learned that we can control much of our own fate through simple but concerted actions in our daily lives such as social distancing, wearing masks, and washing hands. Let's not only remember those facts, but practice them.
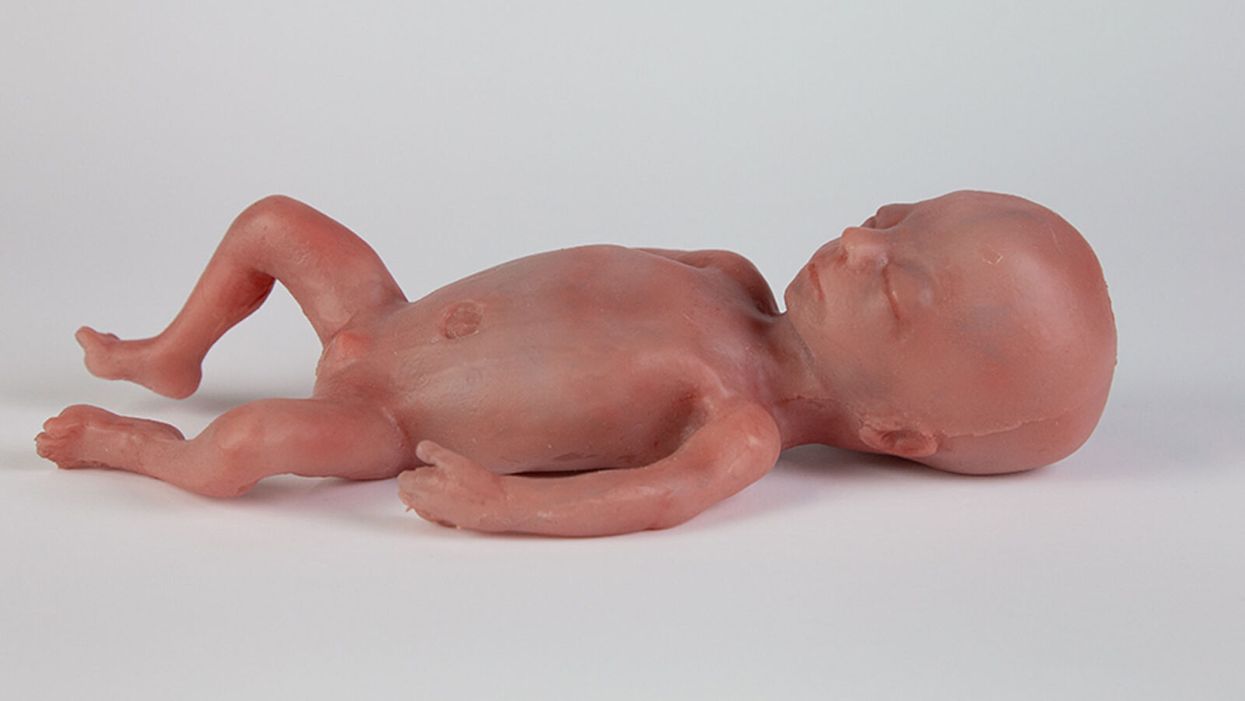
Artificial Wombs Are Getting Closer to Reality for Premature Babies
A mannequin of a 24-week-old fetus replicated from MR imaging. Created by: Juliette van Haren, Mark Thielen, Jasper Sterk, Chet Bangaru, and Frank Delbressine, Department of Industrial Design, Eindhoven University of Technology.
In 2017, researchers at the Children's Hospital of Philadelphia grew extremely preterm lambs from hairless to fluffy inside a "biobag," a dark, fluid-filled bag designed to mimic a mother's womb.
"There could be quite a lot of infants that would benefit from artificial womb technologies."
This happened over the course of a month, across a delicate period of fetal development that scientists consider the "edge of viability" for survival at birth.
In 2019, Australian and Japanese scientists repeated the success of keeping extremely premature lambs inside an artificial womb environment until they were ready to survive on their own. Those researchers are now developing a treatment strategy for infants born at "the hard limit of viability," between 20 and 23 weeks of gestation. At the same time, Dutch researchers are going so far as to replicate the sound of a mother's heartbeat inside a biobag. These developments signal exciting times ahead--with a touch of science fiction--for artificial womb technologies. But is there a catch?
"There could be quite a lot of infants that would benefit from artificial womb technologies," says Josephine Johnston, a bioethicist and lawyer at The Hastings Center, an independent bioethics research institute in New York. "These technologies can decrease morbidity and mortality for infants at the edge of viability and help them survive without significant damage to the lungs or other problems," she says.
It is a viewpoint shared by Frans van de Vosse, leader of the Cardiovascular Biomechanics research group at Eindhoven University of Technology in the Netherlands. He participates in a university project that recently received more than $3 million in funding from the E.U. to produce a prototype artificial womb for preterm babies between 24 and 28 weeks of gestation by 2024.
The Eindhoven design comes with a fluid-based environment, just like that of the natural womb, where the baby receives oxygen and nutrients through an artificial placenta that is connected to the baby's umbilical cord. "With current incubators, when a respiratory device delivers oxygen into the lungs in order for the baby to breathe, you may harm preterm babies because their lungs are not yet mature for that," says van de Vosse. "But when the lungs are under water, then they can develop, they can mature, and the baby will receive the oxygen through the umbilical cord, just like in the natural womb," he says.
His research team is working to achieve the "perfectly natural" artificial womb based on strict mathematical models and calculations, van de Vosse says. They are even employing 3D printing technology to develop the wombs and artificial babies to test in them--the mannequins, as van de Vosse calls them. These mannequins are being outfitted with sensors that can replicate the environment a fetus experiences inside a mother's womb, including the soothing sound of her heartbeat.
"The Dutch study's artificial womb design is slightly different from everything else we have seen as it encourages a gestateling to experience the kind of intimacy that a fetus does in pregnancy," says Elizabeth Chloe Romanis, an assistant professor in biolaw at Durham Law School in the U.K. But what is a "gestateling" anyway? It's a term Romanis has coined to describe neither a fetus nor a newborn, but an in-between artificial stage.
"Because they aren't born, they are not neonates," Romanis explains. "But also, they are not inside a pregnant person's body, so they are not fetuses. In an artificial womb the fetus is still gestating, hence why I call it gestateling."
The terminology is not just a semantic exercise to lend a name to what medical dictionaries haven't yet defined. "Gestatelings might have a slightly different psychology," says Romanis. "A fetus inside a mother's womb interacts with the mother. A neonate has some kind of self-sufficiency in terms of physiology. But the gestateling doesn't do either of those things," she says, urging us to be mindful of the still-obscure effects that experiencing early life as a gestateling might have on future humans. Psychology aside, there are also legal repercussions.
The Universal Declaration of Human Rights proclaims the "inalienable rights which everyone is entitled to as a human being," with "everyone" including neonates. However, such a legal umbrella is absent when it comes to fetuses, which have no rights under the same declaration. "We might need a new legal category for a gestateling," concludes Romanis.
But not everyone agrees. "However well-meaning, a new legal category would almost certainly be used to further erode the legality of abortion in countries like the U.S.," says Johnston.
The "abortion war" in the U.S. has risen to a crescendo since 2019, when states like Missouri, Mississippi, Kentucky, Louisiana and Georgia passed so-called "fetal heartbeat bills," which render an abortion illegal once a fetal heartbeat is detected. The situation is only bound to intensify now that Justice Ruth Bader Ginsburg, one of the Supreme Court's fiercest champions for abortion rights, has passed away. If President Trump appoints Ginsburg's replacement, he will probably grant conservatives on the Court the votes needed to revoke or weaken Roe v. Wade, the milestone decision of 1973 that established women's legal right to an abortion.
"A gestateling with intermediate status would almost certainly be considered by some in the U.S. (including some judges) to have at least certain legal rights, likely including right-to-life," says Johnston. This would enable a fetus on the edge of viability to make claims on the mother, and lead either to a shortening of the window in which abortion is legal—or a practice of denying abortion altogether. Instead, Johnston predicts, doctors might offer to transfer the fetus to an artificial womb for external gestation as a new standard of care.
But the legal conundrum does not stop there. The viability threshold is an estimate decided by medical professionals based on the clinical evidence and the technology available. It is anything but static. In the 1970s when Roe v. Wade was decided, for example, a fetus was considered legally viable starting at 28 weeks. Now, with improved technology and medical management, "the hard limit today is probably 20 or 21 weeks," says Matthew Kemp, associate professor at the University of Western Australia and one of the Australian-Japanese artificial womb project's senior researchers.
The changing threshold can result in situations where lots of people invested in the decision disagree. "Those can be hard decisions, but they are case-by-case decisions that families make or parents make with the key providers to determine when to proceed and when to let the infant die. Usually, it's a shared decision where the parents have the final say," says Johnston. But this isn't always the case.
On May 9th 2016, a boy named Alfie Evans was born in Liverpool, UK. Suffering seizures a few months after his birth, Alfie was diagnosed with an unknown neurodegenerative disorder and soon went into a semi-vegetative state, which lasted for more than a year. Alfie's medical team decided to withdraw his ventilation support, suggesting further treatment was unlawful and inhumane, but his parents wanted permission to fly him to a hospital in Rome and attempt to prolong his life there. In the end, the case went all the way up to the Supreme Court, which ruled that doctors could stop providing life support for Alfie, saying that the child required "peace, quiet and privacy." What happened to little Alfie raised huge publicity in the UK and pointedly highlighted the dilemma of whether parents or doctors should have the final say in the fate of a terminally-ill child in life-support treatment.
"In a few years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue."
Alfie was born and, thus had legal rights, yet legal and ethical mayhem arose out of his case. When it comes to gestatelings, the scenarios will be even more complicated, says Romanis. "I think there's a really big question about who has parental rights and who doesn't," she says. "The assisted reproductive technology (ART) law in the U.K. hasn't been updated since 2008....It certainly needs an update when you think about all the things we have done since [then]."
This June, for instance, scientists from the Wake Forest Institute for Regenerative Medicine in North Carolina published research showing that they could take a small sample of tissue from a rabbit's uterus and create a bioengineered uterus, which then supported both fertilization and normal pregnancy like a natural uterus does.
"In [a number of] years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue," says Dr. Anthony Atala, the Institute's director and a pioneer in regenerative medicine. These bioengineered uteri will eventually be covered by insurance, Atala expects. But when it comes to artificial wombs that externally gestate premature infants, will all mothers have equal access?
Medical reports have already shown racial and ethnic disparities in infertility treatments and access to assisted reproductive technologies. Costs on average total $12,400 per cycle of treatment and may require several cycles to achieve a live birth. "There's no indication that artificial wombs would be treated any differently. That's what we see with almost every expensive new medical technology," says Johnston. In a much more dystopian future, there is even a possibility that inequity in healthcare might create disturbing chasms in how women of various class levels bear children. Romanis asks us to picture the following scenario:
We live in a world where artificial wombs have become mainstream. Most women choose to end their pregnancies early and transfer their gestatelings to the care of machines. After a while, insurers deem full-term pregnancy and childbirth a risky non-necessity, and are lobbying to stop covering them altogether. Wealthy white women continue opting out of their third trimesters (at a high cost), since natural pregnancy has become a substandard route for poorer women. Those women are strongly judged for any behaviors that could risk their fetus's health, in contrast with the machine's controlled environment. "Why are you having a coffee during your pregnancy?" critics might ask. "Why are you having a glass of red wine? If you can't be perfect, why don't you have it the artificial way?"
Problem is, even if they want to, they won't be able to afford it.
In a more sanguine version, however, the artificial wombs are only used in cases of prematurity as a life-saving medical intervention rather than as a lifestyle accommodation. The 15 million babies who are born prematurely each year and may face serious respiratory, cardiovascular, visual and hearing problems, as well as learning disabilities, instead continue their normal development in artificial wombs. After lots of deliberation, insurers agree to bear the cost of external wombs because they are cheaper than a lifetime of medical care for a disabled or diseased person. This enables racial and ethnic minority women, who make up the majority of women giving premature birth, to access the technology.
Even extremely premature babies, those babies (far) below the threshold of 28 weeks of gestation, half of which die, could now discover this thing called life. In this scenario, as the Australian researcher Kemp says, we are simply giving a good shot at healthy, long-term survival to those who were unfortunate enough to start too soon.