Henrietta Lacks' Cells Enabled Medical Breakthroughs. Is It Time to Finally Retire Them?

Henrietta Lacks, (1920-1951) unknowingly had her cells cultured and used in medical research.
For Victoria Tokarz, a third-year PhD student at the University of Toronto, experimenting with cells is just part of a day's work. Tokarz, 26, is studying to be a cell biologist and spends her time inside the lab manipulating muscle cells sourced from rodents to try to figure out how they respond to insulin. She hopes this research could someday lead to a breakthrough in our understanding of diabetes.
"People like to use HeLa cells because they're easy to use."
But in all her research, there is one cell culture that Tokarz refuses to touch. The culture is called HeLa, short for Henrietta Lacks, named after the 31-year-old tobacco farmer the cells were stolen from during a tumor biopsy she underwent in 1951.
"In my opinion, there's no question or experiment I can think of that validates stealing from and profiting off of a black woman's body," Tokarz says. "We're not talking about a reagent we created in a lab, a mixture of some chemicals. We're talking about a human being who suffered indescribably so we could profit off of her misfortune."
Lacks' suffering is something that, until recently, was not widely known. Born to a poor family in Roanoke, VA, Lacks was sent to live with her grandfather on the family tobacco farm at age four, shortly after the death of her mother. She gave birth to her first child at just fourteen, and two years later had another child with profound developmental disabilities. Lacks married her first cousin, David, in 1941 and the family moved to Maryland where they had three additional children.
But the real misfortune came in 1951, when Lacks told her cousins that she felt a hard "knot" in her womb. When Lacks went to Johns Hopkins hospital to have the knot examined, doctors discovered that the hard lump Henrietta felt was a rapidly-growing cervical tumor.
Before the doctors treated the tumor – inserting radium tubes into her vagina, in the hopes they could kill the cancer, Lacks' doctors clipped two tissue samples from her cervix, without Lacks' knowledge or consent. While it's considered widely unethical today, taking tissue samples from patients was commonplace at the time. The samples were sent to a cancer researcher at Johns Hopkins and Lacks continued treatment unsuccessfully until she died a few months later of metastatic cancer.
Lacks' story was not over, however: When her tissue sample arrived at the lab of George Otto Gey, the Johns Hopkins cancer researcher, he noticed that the cancerous cells grew at a shocking pace. Unlike other cell cultures that would die within a day or two of arriving at the lab, Lacks' cells kept multiplying. They doubled every 24 hours, and to this day, have never stopped.
Scientists would later find out that this growth was due to an infection of Human Papilloma Virus, or HPV, which is known for causing aggressive cancers. Lacks' cells became the world's first-ever "immortalized" human cell line, meaning that as long as certain environmental conditions are met, the cells can replicate indefinitely. Although scientists have cultivated other immortalized cell lines since then, HeLa cells remain a favorite among scientists due to their resilience, Tokarz says.
"People like to use HeLa cells because they're easy to use," Tokarz says. "They're easy to manipulate, because they're very hardy, and they allow for transection, which means expressing a protein in a cell that's not normally there. Other cells, like endothelial cells, don't handle those manipulations well."
Once the doctors at Johns Hopkins discovered that Lacks' cells could replicate indefinitely, they started shipping them to labs around the world to promote medical research. As they were the only immortalized cell line available at the time, researchers used them for thousands of experiments — some of which resulted in life-saving treatments. Jonas Salk's polio vaccine, for example, was manufactured using HeLa cells. HeLa cell research was also used to develop a vaccine for HPV, and for the development of in vitro fertilization and gene mapping. Between 1951 and 2018, HeLa cells have been cited in over 110,000 publications, according to a review from the National Institutes of Health.
But while some scientists like Tokarz are thankful for the advances brought about by HeLa cells, they still believe it's well past time to stop using them in research.
"Am I thankful we have a polio vaccine? Absolutely. Do I resent the way we came to have that vaccine? Absolutely," Tokarz says. "We could have still arrived at those same advances by treating her as the human being she is, not just a specimen."
Ethical considerations aside, HeLa is no longer the world's only available cell line – nor, Tokarz argues, are her cells the most suitable for every type of research. "The closer you can get to the physiology of the thing you're studying, the better," she says. "Now we have the ability to use primary cells, which are isolated from a person and put right into the culture dish, and those don't have the same mutations as cells that have been growing for 20 years. We didn't have the expertise to do that initially, but now we do."
Raphael Valdivia, a professor of molecular genetics and microbiology at Duke University School of Medicine, agrees that HeLa cells are no longer optimal for most research. "A lot of scientists are moving away from HeLa cells because they're so unstable," he says. "They mutate, they rearrange chromosomes to become adaptive, and different batches of cells evolve separately from each other. The HeLa cells in my lab are very different than the ones down the hall, and that means sometimes we can't replicate our results. We have to go back to an earlier batch of cells in the freezer and re-test."
Still, the idea of retiring the cells completely doesn't make sense, Valdivia says: "To some extent, you're beholden to previous research. You need to be able to confirm findings that happen in earlier studies, and to do that you need to use the same cell line that other researchers have used."
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice."
"The way in which the cells were taken – without patient consent – is completely inappropriate," says Yann Joly, associate professor at the Faculty of Medicine in Toronto and Research Director at the Centre of Genomics and Policy. "The question now becomes, what can we do about it now? What are our options?"
While scientists are not able to erase what was done to Henrietta Lacks, Joly argues that retiring her cells is also non-consensual, assuming – maybe incorrectly – what Henrietta would have wanted, without her input. Additionally, Joly points out that other immortalized human cell lines are fraught with what some people consider to be ethical concerns as well, such as the human embryonic kidney cell line, commonly referred to as HEK-293, that was derived from an aborted female fetus. "Just because you're using another kind of cell doesn't mean it's devoid of ethical issue," he says.
Seemingly, the one thing scientists can agree on is that Henrietta Lacks was mistreated by the medical community. But even so, retiring her cells from medical research is not an obvious solution. Scientists are now using HeLa cells to better understand how the novel coronavirus affects humans, and this knowledge will inform how researchers develop a COVID-19 vaccine.
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice," Joly says. "If [ethics] were that easy, nobody would need to teach it."
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
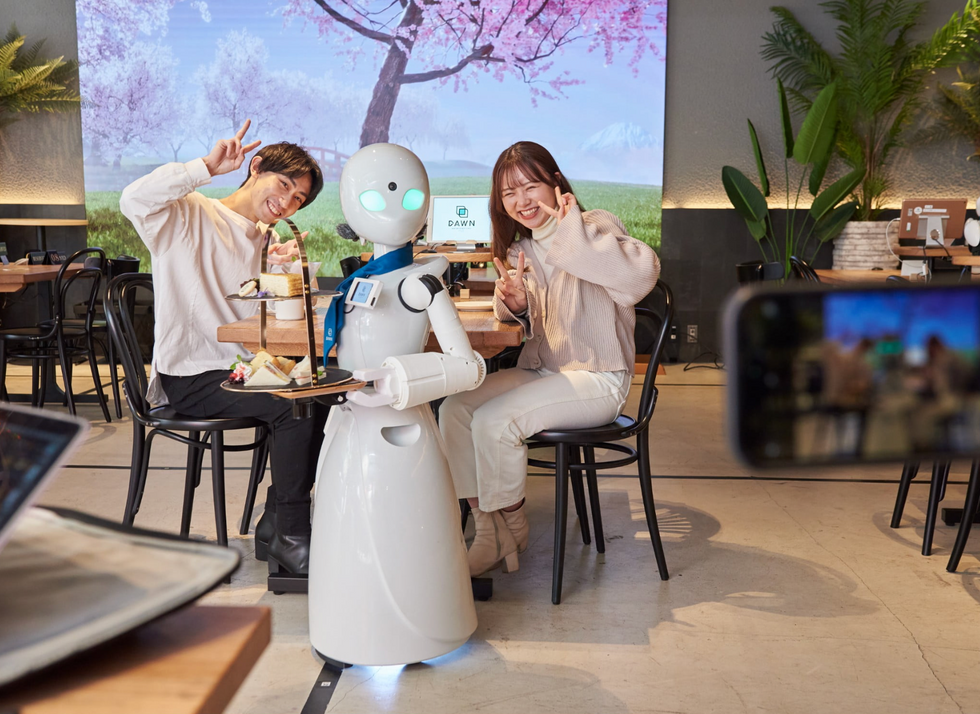
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
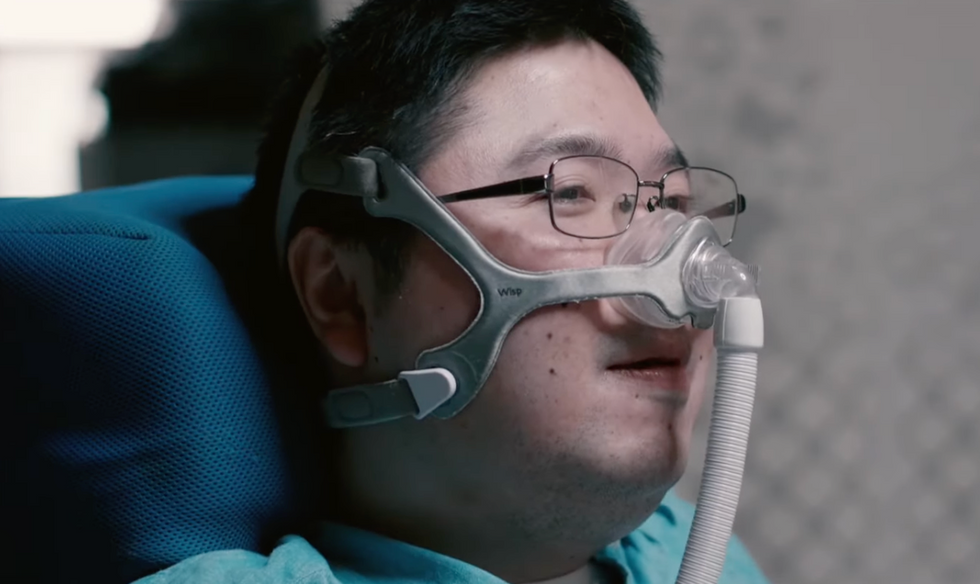
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

