If New Metal Legs Let You Run 20 Miles/Hour, Would You Amputate Your Own?
A patient with below-knee AMI amputation walks up the stairs.
"Here's a question for you," I say to our dinner guests, dodging a knowing glance from my wife. "Imagine a future in which you could surgically replace your legs with robotic substitutes that had all the functionality and sensation of their biological counterparts. Let's say these new legs would allow you to run all day at 20 miles per hour without getting tired. Would you have the surgery?"
Why are we so married to the arbitrary distinction between rehabilitating and augmenting?
Like most people I pose this question to, our guests respond with some variation on the theme of "no way"; the idea of undergoing a surgical procedure with the sole purpose of augmenting performance beyond traditional human limits borders on the unthinkable.
"Would your answer change if you had arthritis in your knees?" This is where things get interesting. People think differently about intervention when injury or illness is involved. The idea of a major surgery becomes more tractable to us in the setting of rehabilitation.
Consider the simplistic example of human walking speed. The average human walks at a baseline three miles per hour. If someone is only able to walk at one mile per hour, we do everything we can to increase their walking ability. However, to take a person who is already able to walk at three miles per hour and surgically alter their body so that they can walk twice as fast seems, to us, unreasonable.
What fascinates me about this is that the three-mile-per-hour baseline is set by arbitrary limitations of the healthy human body. If we ignore this reference point altogether, and consider that each case simply offers an improvement in walking ability, the line between augmentation and rehabilitation all but disappears. Why, then, are we so married to this arbitrary distinction between rehabilitating and augmenting? What makes us hold so tightly to baseline human function?
Where We Stand Now
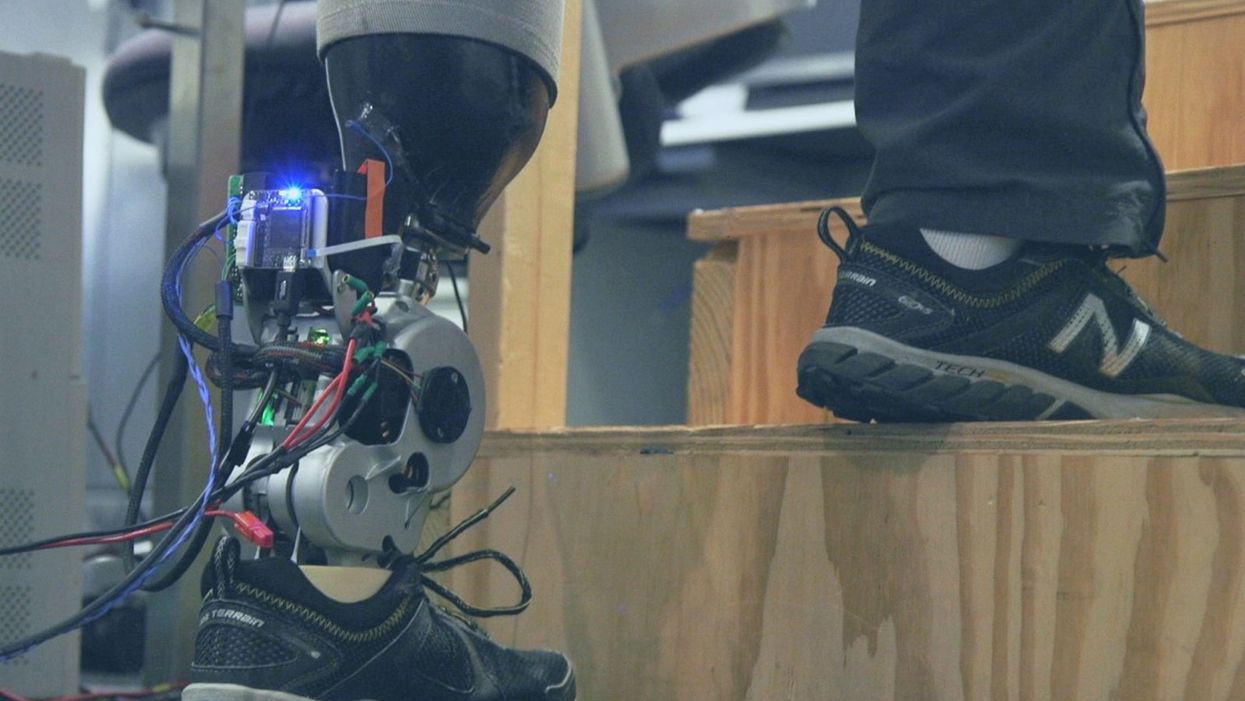
As the functionality of advanced prosthetic devices continues to increase at an astounding rate, questions like these are becoming more relevant. Experimental prostheses, intended for the rehabilitation of people with amputation, are now able to replicate the motions of biological limbs with high fidelity. Neural interfacing technologies enable a person with amputation to control these devices with their brain and nervous system. Before long, synthetic body parts will outperform biological ones.
Our approach allows people to not only control a prosthesis with their brain, but also to feel its movements as if it were their own limb.
Against this backdrop, my colleagues and I developed a methodology to improve the connection between the biological body and a synthetic limb. Our approach, known as the agonist-antagonist myoneural interface ("AMI" for short), enables us to reflect joint movement sensations from a prosthetic limb onto the human nervous system. In other words, the AMI allows people to not only control a prosthesis with their brain, but also to feel its movements as if it were their own limb. The AMI involves a reimagining of the amputation surgery, so that the resultant residual limb is better suited to interact with a neurally-controlled prosthesis. In addition to increasing functionality, the AMI was designed with the primary goal of enabling adoption of a prosthetic limb as part of a patient's physical identity (known as "embodiment").
Early results have been remarkable. Patients with below-knee AMI amputation are better able to control an experimental prosthetic leg, compared to people who had their legs amputated in the traditional way. In addition, the AMI patients show increased evidence of embodiment. They identify with the device, and describe feeling as though it is part of them, part of self.
Where We're Going
True embodiment of robotic devices has the potential to fundamentally alter humankind's relationship with the built world. Throughout history, humans have excelled as tool builders. We innovate in ways that allow us to design and augment the world around us. However, tools for augmentation are typically external to our body identity; there is a clean line drawn between smart phone and self. As we advance our ability to integrate synthetic systems with physical identity, humanity will have the capacity to sculpt that very identity, rather than just the world in which it exists.
For this potential to be realized, we will need to let go of our reservations about surgery for augmentation. In reality, this shift has already begun. Consider the approximately 17.5 million surgical and minimally invasive cosmetic procedures performed in the United States in 2017 alone. Many of these represent patients with no demonstrated medical need, who have opted to undergo a surgical procedure for the sole purpose of synthetically enhancing their body. The ethical basis for such a procedure is built on the individual perception that the benefits of that procedure outweigh its costs.
At present, it seems absurd that amputation would ever reach this point. However, as robotic technology improves and becomes more integrated with self, the balance of cost and benefit will shift, lending a new perspective on what now seems like an unfathomable decision to electively amputate a healthy limb. When this barrier is crossed, we will collide head-on with the question of whether it is acceptable for a person to "upgrade" such an essential part of their body.
At a societal level, the potential benefits of physical augmentation are far-reaching. The world of robotic limb augmentation will be a world of experienced surgeons whose hands are perfectly steady, firefighters whose legs allow them to kick through walls, and athletes who never again have to worry about injury. It will be a world in which a teenage boy and his grandmother embark together on a four-hour sprint through the woods, for the sheer joy of it. It will be a world in which the human experience is fundamentally enriched, because our bodies, which play such a defining role in that experience, are truly malleable.
This is not to say that such societal benefits stand without potential costs. One justifiable concern is the misuse of augmentative technologies. We are all quite familiar with the proverbial supervillain whose nervous system has been fused to that of an all-powerful robot.
The world of robotic limb augmentation will be a world of experienced surgeons whose hands are perfectly steady.
In reality, misuse is likely to be both subtler and more insidious than this. As with all new technology, careful legislation will be necessary to work against those who would hijack physical augmentations for violent or oppressive purposes. It will also be important to ensure broad access to these technologies, to protect against further socioeconomic stratification. This particular issue is helped by the tendency of the cost of a technology to scale inversely with market size. It is my hope that when robotic augmentations are as ubiquitous as cell phones, the technology will serve to equalize, rather than to stratify.
In our future bodies, when we as a society decide that the benefits of augmentation outweigh the costs, it will no longer matter whether the base materials that make us up are biological or synthetic. When our AMI patients are connected to their experimental prosthesis, it is irrelevant to them that the leg is made of metal and carbon fiber; to them, it is simply their leg. After our first patient wore the experimental prosthesis for the first time, he sent me an email that provides a look at the immense possibility the future holds:
What transpired is still slowly sinking in. I keep trying to describe the sensation to people. Then this morning my daughter asked me if I felt like a cyborg. The answer was, "No, I felt like I had a foot."
Gene Transfer Leads to Longer Life and Healthspan
In August, a study provided the first proof-of-principle that genetic material transferred from one species to another can increase both longevity and healthspan in the recipient animal.
The naked mole rat won’t win any beauty contests, but it could possibly win in the talent category. Its superpower: fighting the aging process to live several times longer than other animals its size, in a state of youthful vigor.
It’s believed that naked mole rats experience all the normal processes of wear and tear over their lifespan, but that they’re exceptionally good at repairing the damage from oxygen free radicals and the DNA errors that accumulate over time. Even though they possess genes that make them vulnerable to cancer, they rarely develop the disease, or any other age-related disease, for that matter. Naked mole rats are known to live for over 40 years without any signs of aging, whereas mice live on average about two years and are highly prone to cancer.
Now, these remarkable animals may be able to share their superpower with other species. In August, a study provided what may be the first proof-of-principle that genetic material transferred from one species can increase both longevity and healthspan in a recipient animal.
There are several theories to explain the naked mole rat’s longevity, but the one explored in the study, published in Nature, is based on the abundance of large-molecule high-molecular mass hyaluronic acid (HMM-HA).
A small molecule version of hyaluronic acid is commonly added to skin moisturizers and cosmetics that are marketed as ways to keep skin youthful, but this version, just applied to the skin, won’t have a dramatic anti-aging effect. The naked mole rat has an abundance of the much-larger molecule, HMM-HA, in the chemical-rich solution between cells throughout its body. But does the HMM-HA actually govern the extraordinary longevity and healthspan of the naked mole rat?
To answer this question, Dr. Vera Gorbunova, a professor of biology and oncology at the University of Rochester, and her team created a mouse model containing the naked mole rat gene hyaluronic acid synthase 2, or nmrHas2. It turned out that the mice receiving this gene during their early developmental stage also expressed HMM-HA.
The researchers found that the effects of the HMM-HA molecule in the mice were marked and diverse, exceeding the expectations of the study’s co-authors. High-molecular mass hyaluronic acid was more abundant in kidneys, muscles and other organs of the Has2 mice compared to control mice.
In addition, the altered mice had a much lower incidence of cancer. Seventy percent of the control mice eventually developed cancer, compared to only 57 percent of the altered mice, even after several techniques were used to induce the disease. The biggest difference occurred in the oldest mice, where the cancer incidence for the Has2 mice and the controls was 47 percent and 83 percent, respectively.
With regard to longevity, Has2 males increased their lifespan by more than 16 percent and the females added 9 percent. “Somehow the effect is much more pronounced in male mice, and we don’t have a perfect answer as to why,” says Dr. Gorbunova. Another improvement was in the healthspan of the altered mice: the number of years they spent in a state of relative youth. There’s a frailty index for mice, which includes body weight, mobility, grip strength, vision and hearing, in addition to overall conditions such as the health of the coat and body temperature. The Has2 mice scored lower in frailty than the controls by all measures. They also performed better in tests of locomotion and coordination, and in bone density.
Gorbunova’s results show that a gene artificially transferred from one species can have a beneficial effect on another species for longevity, something that had never been demonstrated before. This finding is “quite spectacular,” said Steven Austad, a biologist at the University of Alabama at Birmingham, who was not involved in the study.
Just as in lifespan, the effects in various organs and systems varied between the sexes, a common occurrence in longevity research, according to Austad, who authored the book Methuselah’s Zoo and specializes in the biological differences between species. “We have ten drugs that we can give to mice to make them live longer,” he says, “and all of them work better in one sex than in the other.” This suggests that more attention needs to be paid to the different effects of anti-aging strategies between the sexes, as well as gender differences in healthspan.
According to the study authors, the HMM-HA molecule delivered these benefits by reducing inflammation and senescence (cell dysfunction and death). The molecule also caused a variety of other benefits, including an upregulation of genes involved in the function of mitochondria, the powerhouses of the cells. These mechanisms are implicated in the aging process, and in human disease. In humans, virtually all noncommunicable diseases entail an acceleration of the aging process.
So, would the gene that creates HMM-HA have similar benefits for longevity in humans? “We think about these questions a lot,” Gorbunova says. “It’s been done by injections in certain patients, but it has a local effect in the treatment of organs affected by disease,” which could offer some benefits, she added.
“Mice are very short-lived and cancer-prone, and the effects are small,” says Steven Austad, a biologist at the University of Alabama at Birmingham. “But they did live longer and stay healthy longer, which is remarkable.”
As for a gene therapy to introduce the nmrHas2 gene into humans to obtain a global result, she’s skeptical because of the complexity involved. Gorbunova notes that there are potential dangers in introducing an animal gene into humans, such as immune responses or allergic reactions.
Austad is equally cautious about a gene therapy. “What this study says is that you can take something a species does well and transfer at least some of that into a new species. It opens up the way, but you may need to transfer six or eight or ten genes into a human” to get the large effect desired. Humans are much more complex and contain many more genes than mice, and all systems in a biological organism are intricately connected. One naked mole rat gene may not make a big difference when it interacts with human genes, metabolism and physiology.
Still, Austad thinks the possibilities are tantalizing. “Mice are very short-lived and cancer-prone, and the effects are small,” he says. “But they did live longer and stay healthy longer, which is remarkable.”
As for further research, says Austad, “The first place to look is the skin” to see if the nmrHas2 gene and the HMM-HA it produces can reduce the chance of cancer. Austad added that it would be straightforward to use the gene to try to prevent cancer in skin cells in a dish to see if it prevents cancer. It would not be hard to do. “We don’t know of any downsides to hyaluronic acid in skin, because it’s already used in skin products, and you could look at this fairly quickly.”
“Aging mechanisms evolved over a long time,” says Gorbunova, “so in aging there are multiple mechanisms working together that affect each other.” All of these processes could play a part and almost certainly differ from one species to the next.
“HMM-HA molecules are large, but we’re now looking for a small-molecule drug that would slow it’s breakdown,” she says. “And we’re looking for inhibitors, now being tested in mice, that would hinder the breakdown of hyaluronic acid.” Gorbunova has found a natural, plant-based product that acts as an inhibitor and could potentially be taken as a supplement. Ultimately, though, she thinks that drug development will be the safest and most effective approach to delivering HMM-HA for anti-aging.
A new study provides key insights in what causes Alzheimer's: a breakdown in the brain’s system for clearing waste.
In recent years, researchers of Alzheimer’s have made progress in figuring out the complex factors that lead to the disease. Yet, the root cause, or causes, of Alzheimer’s are still pretty much a mystery.
In fact, many people get Alzheimer’s even though they lack the gene variant we know can play a role in the disease. This is a critical knowledge gap for research to address because the vast majority of Alzheimer’s patients don’t have this variant.
A new study provides key insights into what’s causing the disease. The research, published in Nature Communications, points to a breakdown over time in the brain’s system for clearing waste, an issue that seems to happen in some people as they get older.
Michael Glickman, a biologist at Technion – Israel Institute of Technology, helped lead this research. I asked him to tell me about his approach to studying how this breakdown occurs in the brain, and how he tested a treatment that has potential to fix the problem at its earliest stages.
Dr. Michael Glickman is internationally renowned for his research on the ubiquitin-proteasome system (UPS), the brain's system for clearing the waste that is involved in diseases such as Huntington's, Alzheimer's, and Parkinson's. He is the head of the Lab for Protein Characterization in the Faculty of Biology at the Technion – Israel Institute of Technology. In the lab, Michael and his team focus on protein recycling and the ubiquitin-proteasome system, which protects against serious diseases like Alzheimer’s, Parkinson’s, cystic fibrosis, and diabetes. After earning his PhD at the University of California at Berkeley in 1994, Michael joined the Technion as a Senior Lecturer in 1998 and has served as a full professor since 2009.

Dr. Michael Glickman

