How 30 Years of Heart Surgeries Taught My Dad How to Live

A mid-1970s photo of the author's father, and him holding a grandchild in 2012.
[Editor's Note: This piece is the winner of our 2019 essay contest, which prompted readers to reflect on the question: "How has an advance in science or medicine changed your life?"]
My father did not expect to live past the age of 50. Neither of his parents had done so. And he also knew how he would die: by heart attack, just as his father did.
In July of 1976, he had his first heart attack, days before his 40th birthday.
My dad lived the first 40 years of his life with this knowledge buried in his bones. He started smoking at the age of 12, and was drinking before he was old enough to enlist in the Navy. He had a sarcastic, often cruel, sense of humor that could drive my mother, my sister and me into tears. He was not an easy man to live with, but that was okay by him - he didn't expect to live long.
In July of 1976, he had his first heart attack, days before his 40th birthday. I was 13, and my sister was 11. He needed quadruple bypass surgery. Our small town hospital was not equipped to do this type of surgery; he would have to be transported 40 miles away to a heart center. I understood this journey to mean that my father was seriously ill, and might die in the hospital, away from anyone he knew. And my father knew a lot of people - he was a popular high school English teacher, in a town with only three high schools. He knew generations of students and their parents. Our high school football team did a blood drive in his honor.

During a trip to Disney World in 1974, Dad was suffering from angina the entire time but refused to tell me (left) and my sister, Kris.
Quadruple bypass surgery in 1976 meant that my father's breastbone was cut open by a sternal saw. His ribcage was spread wide. After the bypass surgery, his bones would be pulled back together, and tied in place with wire. The wire would later be pulled out of his body when the bones knitted back together. It would take months before he was fully healed.
Dad was in the hospital for the rest of the summer and into the start of the new school year. Going to visit him was farther than I could ride my bicycle; it meant planning a trip in the car and going onto the interstate. The first time I was allowed to visit him in the ICU, he was lying in bed, and then pushed himself to sit up. The heart monitor he was attached to spiked up and down, and I fainted. I didn't know that heartbeats change when you move; television medical dramas never showed that - I honestly thought that I had driven my father into another heart attack.
Only a few short years after that, my father returned to the big hospital to have his heart checked with a new advance in heart treatment: a CT scan. This would allow doctors to check for clogged arteries and treat them before a fatal heart attack. The procedure identified a dangerous blockage, and my father was admitted immediately. This time, however, there was no need to break bones to get to the problem; my father was home within a month.
During the late 1970's, my father changed none of his habits. He was still smoking, and he continued to drink. But now, he was also taking pills - pills to manage the pain. He would pop a nitroglycerin tablet under his tongue whenever he was experiencing angina (I have a vivid memory of him doing this during my driving lessons), but he never mentioned that he was in pain. Instead, he would snap at one of us, or joke that we were killing him.
I think he finally determined that, if he was going to have these extra decades of life, he wanted to make them count.
Being the kind of guy he was, my father never wanted to talk about his health. Any admission of pain implied that he couldn't handle pain. He would try to "muscle through" his angina, as if his willpower would be stronger than his heart muscle. His efforts would inevitably fail, leaving him angry and ready to lash out at anyone or anything. He would blame one of us as a reason he "had" to take valium or pop a nitro tablet. Dinners often ended in shouts and tears, and my father stalking to the television room with a bottle of red wine.
In the 1980's while I was in college, my father had another heart attack. But now, less than 10 years after his first, medicine had changed: our hometown hospital had the technology to run dye through my father's blood stream, identify the blockages, and do preventative care that involved statins and blood thinners. In one case, the doctors would take blood vessels from my father's legs, and suture them to replace damaged arteries around his heart. New advances in cholesterol medication and treatments for angina could extend my father's life by many years.
My father decided it was time to quit smoking. It was the first significant health step I had ever seen him take. Until then, he treated his heart issues as if they were inevitable, and there was nothing that he could do to change what was happening to him. Quitting smoking was the first sign that my father was beginning to move out of his fatalistic mindset - and the accompanying fatal behaviors that all pointed to an early death.
In 1986, my father turned 50. He had now lived longer than either of his parents. The habits he had learned from them could be changed. He had stopped smoking - what else could he do?
It was a painful decade for all of us. My parents divorced. My sister quit college. I moved to the other side of the country and stopped speaking to my father for almost 10 years. My father remarried, and divorced a second time. I stopped counting the number of times he was in and out of the hospital with heart-related issues.
In the early 1990's, my father reached out to me. I think he finally determined that, if he was going to have these extra decades of life, he wanted to make them count. He traveled across the country to spend a week with me, to meet my friends, and to rebuild his relationship with me. He did the same with my sister. He stopped drinking. He was more forthcoming about his health, and admitted that he was taking an antidepressant. His humor became less cruel and sadistic. He took an active interest in the world. He became part of my life again.
The 1990's was also the decade of angioplasty. My father explained it to me like this: during his next surgery, the doctors would place balloons in his arteries, and inflate them. The balloons would then be removed (or dissolve), leaving the artery open again for blood. He had several of these surgeries over the next decade.
When my father was in his 60's, he danced at with me at my wedding. It was now 10 years past the time he had expected to live, and his life was transformed. He was living with a woman I had known since I was a child, and my wife and I would make regular visits to their home. My father retired from teaching, became an avid gardener, and always had a home project underway. He was a happy man.

Dancing with my father at my wedding in 1998.
Then, in the mid 2000's, my father faced another serious surgery. Years of arterial surgery, angioplasty, and damaged heart muscle were taking their toll. He opted to undergo a life-saving surgery at Cleveland Clinic. By this time, I was living in New York and my sister was living in Arizona. We both traveled to the Midwest to be with him. Dad was unconscious most of the time. We took turns holding his hand in the ICU, encouraging him to regain his will to live, and making outrageous threats if he didn't listen to us.
The nursing staff were wonderful. I remember telling them that my father had never expected to live this long. One of the nurses pointed out that most of the patients in their ward were in their 70's and 80's, and a few were in their 90's. She reminded me that just a decade earlier, most hospitals were unwilling to do the kind of surgery my father had received on patients his age. In the first decade of the 21st century, however, things were different: 90-year-olds could now undergo heart surgery and live another decade. My father was on the "young" side of their patients.
The Cleveland Clinic visit would be the last major heart surgery my father would have. Not that he didn't return to his local hospital a few times after that: he broke his neck -- not once, but twice! -- slipping on ice. And in the 2010's, he began to show signs of dementia, and needed more home care. His partner, who had her own health issues, was not able to provide the level of care my father needed. My sister invited him to move in with her, and in 2015, I traveled with him to Arizona to get him settled in.
After a few months, he accepted home hospice. We turned off his pacemaker when the hospice nurse explained to us that the job of a pacemaker is to literally jolt a patient's heart back into beating. The jolts were happening more and more frequently, causing my Dad additional, unwanted pain.

My father in 2015, a few months before his death.
My father died in February 2016. His body carried the scars and implants of 30 years of cardiac surgeries, from the ugly breastbone scar from the 1970's to scars on his arms and legs from borrowed blood vessels, to the tiny red circles of robotic incisions from the 21st century. The arteries and veins feeding his heart were a patchwork of transplanted leg veins and fragile arterial walls pressed thinner by balloons.
And my father died with no regrets or unfinished business. He died in my sister's home, with his long-time partner by his side. Medical advancements had given him the opportunity to live 30 years longer than he expected. But he was the one who decided how to live those extra years. He was the one who made the years matter.
Beyond Henrietta Lacks: How the Law Has Denied Every American Ownership Rights to Their Own Cells
A 2017 portrait of Henrietta Lacks.
The common perception is that Henrietta Lacks was a victim of poverty and racism when in 1951 doctors took samples of her cervical cancer without her knowledge or permission and turned them into the world's first immortalized cell line, which they called HeLa. The cell line became a workhorse of biomedical research and facilitated the creation of medical treatments and cures worth untold billions of dollars. Neither Lacks nor her family ever received a penny of those riches.
But racism and poverty is not to blame for Lacks' exploitation—the reality is even worse. In fact all patients, then and now, regardless of social or economic status, have absolutely no right to cells that are taken from their bodies. Some have called this biological slavery.
How We Got Here
The case that established this legal precedent is Moore v. Regents of the University of California.
John Moore was diagnosed with hairy-cell leukemia in 1976 and his spleen was removed as part of standard treatment at the UCLA Medical Center. On initial examination his physician, David W. Golde, had discovered some unusual qualities to Moore's cells and made plans prior to the surgery to have the tissue saved for research rather than discarded as waste. That research began almost immediately.
"On both sides of the case, legal experts and cultural observers cautioned that ownership of a human body was the first step on the slippery slope to 'bioslavery.'"
Even after Moore moved to Seattle, Golde kept bringing him back to Los Angeles to collect additional samples of blood and tissue, saying it was part of his treatment. When Moore asked if the work could be done in Seattle, he was told no. Golde's charade even went so far as claiming to find a low-income subsidy to pay for Moore's flights and put him up in a ritzy hotel to get him to return to Los Angeles, while paying for those out of his own pocket.
Moore became suspicious when he was asked to sign new consent forms giving up all rights to his biological samples and he hired an attorney to look into the matter. It turned out that Golde had been lying to his patient all along; he had been collecting samples unnecessary to Moore's treatment and had turned them into a cell line that he and UCLA had patented and already collected millions of dollars in compensation. The market for the cell lines was estimated at $3 billion by 1990.
Moore felt he had been taken advantage of and filed suit to claim a share of the money that had been made off of his body. "On both sides of the case, legal experts and cultural observers cautioned that ownership of a human body was the first step on the slippery slope to 'bioslavery,'" wrote Priscilla Wald, a professor at Duke University whose career has focused on issues of medicine and culture. "Moore could be viewed as asking to commodify his own body part or be seen as the victim of the theft of his most private and inalienable information."
The case bounced around different levels of the court system with conflicting verdicts for nearly six years until the California Supreme Court ruled on July 9, 1990 that Moore had no legal rights to cells and tissue once they were removed from his body.
The court made a utilitarian argument that the cells had no value until scientists manipulated them in the lab. And it would be too burdensome for researchers to track individual donations and subsequent cell lines to assure that they had been ethically gathered and used. It would impinge on the free sharing of materials between scientists, slow research, and harm the public good that arose from such research.
"In effect, what Moore is asking us to do is impose a tort duty on scientists to investigate the consensual pedigree of each human cell sample used in research," the majority wrote. In other words, researchers don't need to ask any questions about the materials they are using.
One member of the court did not see it that way. In his dissent, Stanley Mosk raised the specter of slavery that "arises wherever scientists or industrialists claim, as defendants have here, the right to appropriate and exploit a patient's tissue for their sole economic benefit—the right, in other words, to freely mine or harvest valuable physical properties of the patient's body. … This is particularly true when, as here, the parties are not in equal bargaining positions."
Mosk also cited the appeals court decision that the majority overturned: "If this science has become for profit, then we fail to see any justification for excluding the patient from participation in those profits."
But the majority bought the arguments that Golde, UCLA, and the nascent biotechnology industry in California had made in amici briefs filed throughout the legal proceedings. The road was now cleared for them to develop products worth billions without having to worry about or share with the persons who provided the raw materials upon which their research was based.
Critical Views
Biomedical research requires a continuous and ever-growing supply of human materials for the foundation of its ongoing work. If an increasing number of patients come to feel as John Moore did, that the system is ripping them off, then they become much less likely to consent to use of their materials in future research.
Some legal and ethical scholars say that donors should be able to limit the types of research allowed for their tissues and researchers should be monitored to assure compliance with those agreements. For example, today it is commonplace for companies to certify that their clothing is not made by child labor, their coffee is grown under fair trade conditions, that food labeled kosher is properly handled. Should we ask any less of our pharmaceuticals than that the donors whose cells made such products possible have been treated honestly and fairly, and share in the financial bounty that comes from such drugs?
Protection of individual rights is a hallmark of the American legal system, says Lisa Ikemoto, a law professor at the University of California Davis. "Putting the needs of a generalized public over the interests of a few often rests on devaluation of the humanity of the few," she writes in a reimagined version of the Moore decision that upholds Moore's property claims to his excised cells. The commentary is in a chapter of a forthcoming book in the Feminist Judgment series, where authors may only use legal precedent in effect at the time of the original decision.
"Why is the law willing to confer property rights upon some while denying the same rights to others?" asks Radhika Rao, a professor at the University of California, Hastings College of the Law. "The researchers who invest intellectual capital and the companies and universities that invest financial capital are permitted to reap profits from human research, so why not those who provide the human capital in the form of their own bodies?" It might be seen as a kind of sweat equity where cash strapped patients make a valuable in kind contribution to the enterprise.
The Moore court also made a big deal about inhibiting the free exchange of samples between scientists. That has become much less the situation over the more than three decades since the decision was handed down. Ironically, this decision, as well as other laws and regulations, have since strengthened the power of patents in biomedicine and by doing so have increased secrecy and limited sharing.
"Although the research community theoretically endorses the sharing of research, in reality sharing is commonly compromised by the aggressive pursuit and defense of patents and by the use of licensing fees that hinder collaboration and development," Robert D. Truog, Harvard Medical School ethicist and colleagues wrote in 2012 in the journal Science. "We believe that measures are required to ensure that patients not bear all of the altruistic burden of promoting medical research."
Additionally, the increased complexity of research and the need for exacting standardization of materials has given rise to an industry that supplies certified chemical reagents, cell lines, and whole animals bred to have specific genetic traits to meet research needs. This has been more efficient for research and has helped to ensure that results from one lab can be reproduced in another.
The Court's rationale of fostering collaboration and free exchange of materials between researchers also has been undercut by the changing structure of that research. Big pharma has shrunk the size of its own research labs and over the last decade has worked out cooperative agreements with major research universities where the companies contribute to the research budget and in return have first dibs on any findings (and sometimes a share of patent rights) that come out of those university labs. It has had a chilling effect on the exchange of materials between universities.
Perhaps tracking cell line donors and use restrictions on those donations might have been burdensome to researchers when Moore was being litigated. Some labs probably still kept their cell line records on 3x5 index cards, computers were primarily expensive room-size behemoths with limited capacity, the internet barely existed, and there was no cloud storage.
But that was the dawn of a new technological age and standards have changed. Now cell lines are kept in state-of-the-art sub zero storage units, tagged with the source, type of tissue, date gathered and often other information. Adding a few more data fields and contacting the donor if and when appropriate does not seem likely to disrupt the research process, as the court asserted.
Forging the Future
"U.S. universities are awarded almost 3,000 patents each year. They earn more than $2 billion each year from patent royalties. Sharing a modest portion of these profits is a novel method for creating a greater sense of fairness in research relationships that we think is worth exploring," wrote Mark Yarborough, a bioethicist at the University of California Davis Medical School, and colleagues. That was penned nearly a decade ago and those numbers have only grown.
The Michigan BioTrust for Health might serve as a useful model in tackling some of these issues. Dried blood spots have been collected from all newborns for half a century to be tested for certain genetic diseases, but controversy arose when the huge archive of dried spots was used for other research projects. As a result, the state created a nonprofit organization to in essence become a biobank and manage access to these spots only for specific purposes, and also to share any revenue that might arise from that research.
"If there can be no property in a whole living person, does it stand to reason that there can be no property in any part of a living person? If there were, can it be said that this could equate to some sort of 'biological slavery'?" Irish ethicist Asim A. Sheikh wrote several years ago. "Any amount of effort spent pondering the issue of 'ownership' in human biological materials with existing law leaves more questions than answers."
Perhaps the biggest question will arise when -- not if but when -- it becomes possible to clone a human being. Would a human clone be a legal person or the property of those who created it? Current legal precedent points to it being the latter.
Today, October 4, is the 70th anniversary of Henrietta Lacks' death from cancer. Over those decades her immortalized cells have helped make possible miraculous advances in medicine and have had a role in generating billions of dollars in profits. Surviving family members have spoken many times about seeking a share of those profits in the name of social justice; they intend to file lawsuits today. Such cases will succeed or fail on their own merits. But regardless of their specific outcomes, one can hope that they spark a larger public discussion of the role of patients in the biomedical research enterprise and lead to establishing a legal and financial claim for their contributions toward the next generation of biomedical research.
Is a Successful HIV Vaccine Finally on the Horizon?
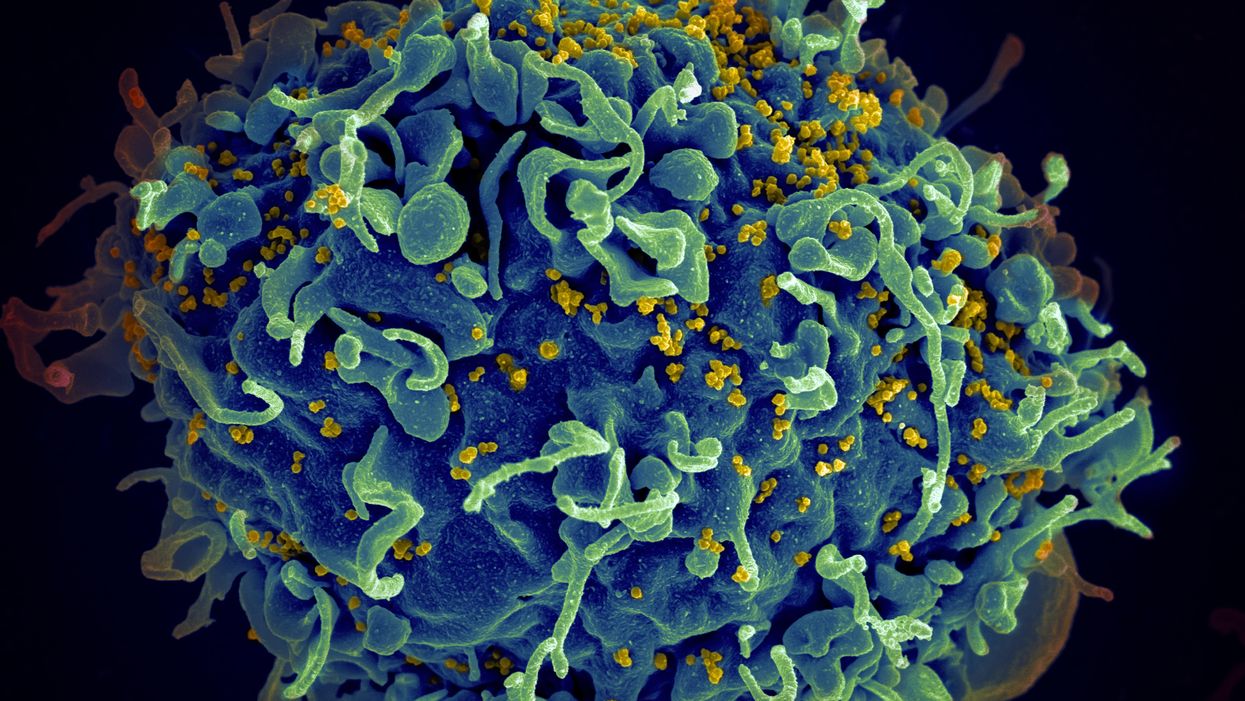
The HIV virus (yellow) infecting a human cell.
Few vaccines have been as complicated—and filled with false starts and crushed hopes—as the development of an HIV vaccine.
While antivirals help HIV-positive patients live longer and reduce viral transmission to virtually nil, these medications must be taken for life, and preventative medications like pre-exposure prophylaxis, known as PrEP, need to be taken every day to be effective. Vaccines, even if they need boosters, would make prevention much easier.
In August, Moderna began human trials for two HIV vaccine candidates based on messenger RNA.
As they have with the Covid-19 pandemic, mRNA vaccines could change the game. The technology could be applied for gene editing therapy, cancer, other infectious diseases—even a universal influenza vaccine.
In the past, three other mRNA vaccines completed phase-2 trials without success. But the easily customizable platforms mean the vaccines can be tweaked better to target HIV as researchers learn more.
Ever since HIV was discovered as the virus causing AIDS, researchers have been searching for a vaccine. But the decades-long journey has so far been fruitless; while some vaccine candidates showed promise in early trials, none of them have worked well among later-stage clinical trials.
There are two main reasons for this: HIV evolves incredibly quickly, and the structure of the virus makes it very difficult to neutralize with antibodies.
"We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
"You know the panic that goes on when a new coronavirus variant surfaces?" asked John Moore, professor of microbiology and immunology at Weill Cornell Medicine who has researched HIV vaccines for 25 years. "With HIV, that kind of variation [happens] pretty much every day in everybody who's infected. It's just orders of magnitude more variable a virus."
Vaccines like these usually work by imitating the outer layer of a virus to teach cells how to recognize and fight off the real thing off before it enters the cell. "If you can prevent landing, you can essentially keep the virus out of the cell," said Larry Corey, the former president and director of the Fred Hutchinson Cancer Research Center who helped run a recent trial of a Johnson & Johnson HIV vaccine candidate, which failed its first efficacy trial.
Like the coronavirus, HIV also has a spike protein with a receptor-binding domain—what Moore calls "the notorious RBD"—that could be neutralized with antibodies. But while that target sticks out like a sore thumb in a virus like SARS-CoV-2, in HIV it's buried under a dense shield. That's not the only target for neutralizing the virus, but all of the targets evolve rapidly and are difficult to reach.
"We understand these targets. We know where they are. But it's still proving incredibly difficult to raise antibodies against them by vaccination," Moore said.
In fact, mRNA vaccines for HIV have been under development for years. The Covid vaccines were built on decades of that research. But it's not as simple as building on this momentum, because of how much more complicated HIV is than SARS-CoV-2, researchers said.
"They haven't succeeded because they were not designed appropriately and haven't been able to induce what is necessary for them to induce," Moore said. "The mRNA technology will enable you to produce a lot of antibodies to the HIV envelope, but if they're the wrong antibodies that doesn't solve the problem."
Part of the problem is that the HIV vaccines have to perform better than our own immune systems. Many vaccines are created by imitating how our bodies overcome an infection, but that doesn't happen with HIV. Once you have the virus, you can't fight it off on your own.
"The human immune system actually does not know how to innately cure HIV," Corey said. "We needed to improve upon the human immune system to make it quicker… with Covid. But we have to actually be better than the human immune system" with HIV.
But in the past few years, there have been impressive leaps in understanding how an HIV vaccine might work. Scientists have known for decades that neutralizing antibodies are key for a vaccine. But in 2010 or so, they were able to mimic the HIV spike and understand how antibodies need to disable the virus. "It helps us understand the nature of the problem, but doesn't instantly solve the problem," Moore said. "Without neutralizing antibodies, you don't have a chance."
Because the vaccines need to induce broadly neutralizing antibodies, and because it's very difficult to neutralize the highly variable HIV, any vaccine will likely be a series of shots that teach the immune system to be on the lookout for a variety of potential attacks.
"Each dose is going to have to have a different purpose," Corey said. "And we hope by the end of the third or fourth dose, we will achieve the level of neutralization that we want."
That's not ideal, because each individual component has to be made and tested—and four shots make the vaccine harder to administer.
"You wouldn't even be going down that route, if there was a better alternative," Moore said. "But there isn't a better alternative."
The mRNA platform is exciting because it is easily customizable, which is especially important in fighting against a shapeshifting, complicated virus. And the mRNA platform has shown itself, in the Covid pandemic, to be safe and quick to make. Effective Covid vaccines were comparatively easy to develop, since the coronavirus is easier to battle than HIV. But companies like Moderna are capitalizing on their success to launch other mRNA therapeutics and vaccines, including the HIV trial.
"You can make the vaccine in two months, three months, in a research lab, and not a year—and the cost of that is really less," Corey said. "It gives us a chance to try many more options, if we've got a good response."
In a trial on macaque monkeys, the Moderna vaccine reduced the chances of infection by 85 percent. "The mRNA platform represents a very promising approach for the development of an HIV vaccine in the future," said Dr. Peng Zhang, who is helping lead the trial at the National Institute of Allergy and Infectious Diseases.
Moderna's trial in humans represents "a very exciting possibility for the prevention of HIV infection," Dr. Monica Gandhi, director of the UCSF-Gladstone Center for AIDS Research, said in an email. "We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
If a successful HIV vaccine is developed, the series of shots could include an mRNA shot that primes the immune system, followed by protein subunits that generate the necessary antibodies, Moore said.
"I think it's the only thing that's worth doing," he said. "Without something complicated like that, you have no chance of inducing broadly neutralizing antibodies."
"I can't guarantee you that's going to work," Moore added. "It may completely fail. But at least it's got some science behind it."