How Bacteria-Killing Viruses May Save Us From Antibiotic Resistance
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
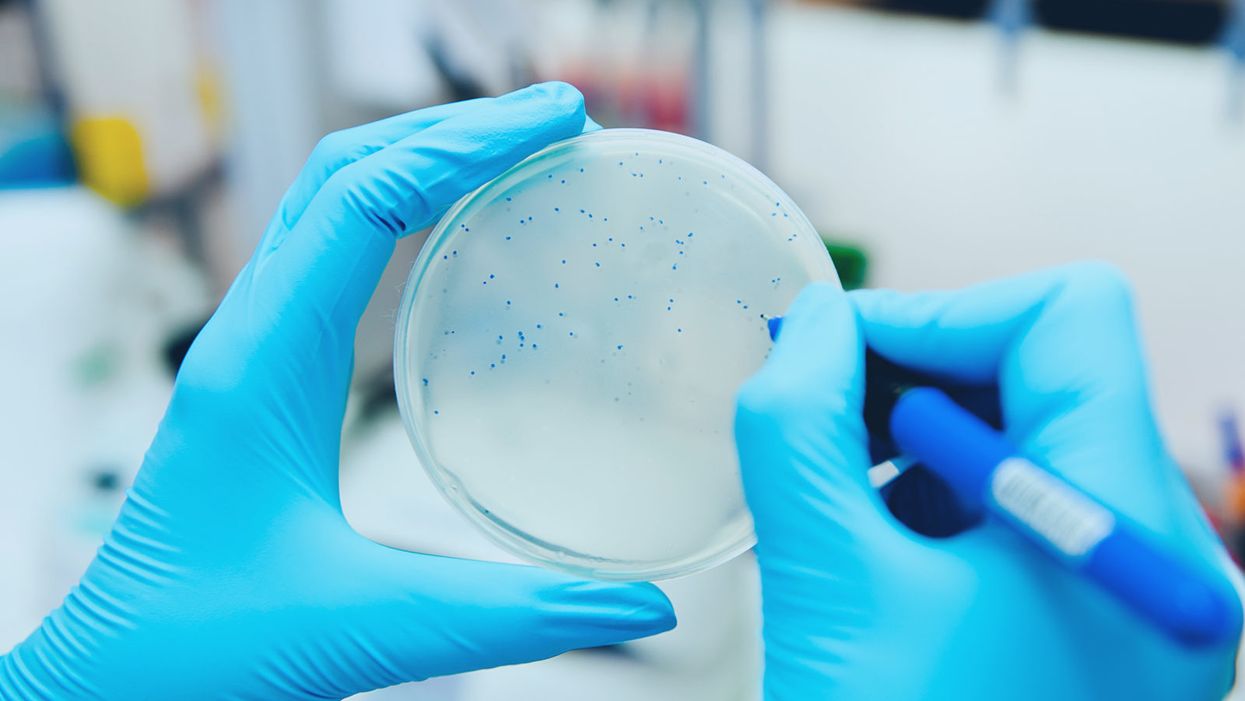
Hand-counting bacteriophage plaques during a titer test.
In my hometown of Pittsburgh, it is not uncommon to read about cutting-edge medical breakthroughs, because Pittsburgh is the home of many innovations in medical science, from the polio vaccine to pioneering organ transplantation. However, medical headlines from Pittsburgh last November weren't heralding a new discovery for once. They were carrying a plea—for a virus.
Phages are weapons of bacterial destruction, but despite recognition of their therapeutic potential for over 100 years, there are zero phage products commercially available to medicine in the United States.
Specifically, a bacteria-killing virus that could attack and control a certain highly drug-resistant bacterial infection ravaging the newly transplanted lungs of a 25-year-old woman named Mallory Smith. The culprit bacteria, Burkholderia cepacia, is a notoriously vicious bacterium that preys on patients with cystic fibrosis who, throughout their life, are exposed to course after course of antibiotics, often fostering a population of highly resistant bacteria that can become too formidable for modern medicine to combat.
What Smith and her physicians desperately needed was a tool that would move beyond failed courses of antibiotics. What they sought was called a bacteriophage. These are naturally occurring ubiquitous viruses that target not humans, but bacteria. The world literally teems with "phages" and one cannot take a bite or drink of anything without encountering them. These weapons of bacterial destruction are exquisitely evolved to target bacteria and, as such, are not harmful to humans. However, despite recognition of their therapeutic potential for over 100 years, there are zero bacteriophage products commercially available to medicine in the United States, at a time when antibiotic resistance is arguably our most pressing public health crisis. Just this week, a new study was published in the Proceedings of the National Academy of Sciences detailing the global scope of the problem.
Why Were These Promising Tools Forgotten?
Phages weren't always relegated to this status. In fact, in the early 20th century phages could be found on American drug store shelves and were used for a variety of ailments. However, the path-breaking discovery and development of antimicrobials agents such as the sulfa drugs and, later the antibiotic penicillin, supplanted the world of phage therapeutics in the United States and many other places.
Fortunately, phage therapy never fully disappeared, and research and clinical use continued in Eastern European nations such as Georgia and Poland.
The antibiotic age revolutionized medicine in a way that arguably no other innovation has. Not only did antibiotics tame many once-deadly infectious diseases, but they made much of modern medicine – from cancer chemotherapy to organ transplantation to joint replacement – possible. Antibiotics, unlike the exquisitely evolved bacteriophage, possessed a broader spectrum of activity and were active against a range of bacteria. This non-specificity facilitated antibiotic use without the need for a specific diagnosis. A physician does not need to know the specific bacterial genus and species causing, for example, a skin infection or pneumonia, but can select an antibiotic that covers the likely culprits and use it empirically, fully expecting the infection to be controlled. Unfortunately, this non-specificity engendered the overuse of antibiotics whose consequences we are now suffering. A bacteriophage, on the other hand, will work against one specific bacterial species and is evolved for just that role.
Phages to the Rescue
As the march of antibiotic resistance has predictably continued since the dawn of the antibiotic age, the prospect of resurrecting phage therapy has been increasingly viewed as one solution. Fortunately, phage therapy never fully disappeared, and research and clinical use continued in Eastern European nations such as Georgia and Poland. However, much of that experience has remained opaque to the medical community at large and questions about dosage, toxicity, efficacy, and method of delivery left many questions without full answers.
Though real questions remained regarding phage use, dire circumstances of prolific antibiotic resistance necessitated their use in the U.S. in two prominent instances involving life-threatening infections. The first case involved an Acinetobacter baumanii infection of the pancreas in a San Diego man in which phages were administered intravenously in 2016. The other case, also in 2016, involved the instillation of phages, fished out of a pond, into the chest cavity of man with a Pseudmonas aeruginosa infection of a prosthetic graft of the aorta. Both cases were successful and were what fueled the Pittsburgh-based plea for Burkholderia phages.
The phages you begin with may not be the ones you end up with, as Darwinian evolutionary pressures will alter the phage in order to keep up with the ongoing evolution of its bacterial target.
How Phages Differ from Other Medical Products
It might seem surprising that in light of the urgent need for new treatments for drug-resistant infections, the pharmaceutical armamentarium is not teeming with phages like a backyard pond. However, phages have been difficult to fit into the current regulatory framework that operates in most developed countries such as the U.S. because of their unique characteristics.
Phages are not one homogenous product like a tablet of penicillin, but a cocktail of viruses that change and evolve as they replicate. The phages you begin with may not be the ones you end up with, as Darwinian evolutionary pressures will alter the phage in order to keep up with the ongoing evolution of its bacterial target. The cocktail may not just contain one specific phage, but a range of phages that all target some specific bacteria in order to increase efficacy. These phage cocktails might also need adjusting to keep pace with bacterial resistance. Additionally, the concentration of phage in a human body after administration is not so easy to predict as phage numbers will rise and fall based on the number of target bacteria that are present.
All of these characteristics make phages very unique when viewed through a regulatory lens, and necessitate the creation of new methods to evaluate them, given that regulatory approval is required. Using phages in the U.S. now requires FDA permission through an investigational new drug application, which can be expedited during an emergency situation. FDA scientists are actively involved in understanding the best means to evaluate bacteriophage therapy and several companies are in early-stage development, though no major clinical trials in the U.S. are currently underway.
One FDA-approved application of phages has seen them used on food products at delis and even in slaughterhouses to diminish the quantity of bacteria on certain meat products.
Would That Humans Were As Lucky As Bologna
Because of the regulatory difficulties with human-use approval, some phage companies have taken another route to develop phage products: food safety. Food safety is a major public health endeavor, and keeping food that people consume safe from E.coli, Listeria, and Salmonella, for example, are rightfully major priorities of industry. One FDA-approved application of phages has seen them used on food products at delis and even in slaughterhouses to diminish the quantity of bacteria on certain meat products.
This use, unlike that for human therapeutic purposes, has found success with regulators: phages, not surprisingly, have been granted the "generally regarded as safe (GRAS)" designation.
A Phage Directory
Tragically Mallory Smith succumbed to her infection despite getting a dose of phages culled from sludge in the Philippines and Fiji. However, her death and last-minute crusade to obtain phages has prompted the call for a phage directory. This directory could catalog the various phages being studied and the particular bacteria they target. Such a searchable index will facilitate the rapid identification and – hopefully – delivery of phages to patients.
If phage therapy is to move from a last-ditch emergency measure to a routine tool for infectious disease physicians, it will be essential that the hurdles they face are eliminated.
Moving Beyond Antibiotics
As we move increasingly toward a post-antibiotic age in infectious disease, moving outside of the traditional paradigm of broad-spectrum antibiotics to non-traditional therapeutics such as bacteriophages and other novel products will become increasingly necessary. Already, clinical trials are underway in various populations, including a major trial in European burn patients.
It is important to understand that there are important scientific and therapeutic questions regarding dose, route of administration and other related questions that need to be addressed before phage use becomes more routine, and it is only through clinical trials conducted with the hope of eventual commercialization that these answers will be found. If phage therapy is to move from a last-ditch emergency measure to a routine tool for infectious disease physicians, it will be essential that the hurdles they face are eliminated.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
New implants let paraplegics surf the web and play computer games
Rodney Gorham, an Australian living with ALS, has reconnected with the world, thanks to a brain-machine interface called the Stentrode.
When I greeted Rodney Gorham, age 63, in an online chat session, he replied within seconds: “My pleasure.”
“Are you moving parts of your body as you type?” I asked.
This time, his response came about five minutes later: “I position the cursor with the eye tracking and select the same with moving my ankles.” Gorham, a former sales representative from Melbourne, Australia, living with amyotrophic lateral sclerosis, or ALS, a rare form of Lou Gehrig’s disease that impairs the brain’s nerve cells and the spinal cord, limiting the ability to move. ALS essentially “locks” a person inside their own body. Gorham is conversing with me by typing with his mind only–no fingers in between his brain and his computer.
The brain-computer interface enabling this feat is called the Stentrode. It's the brainchild of Synchron, a company backed by Amazon’s Jeff Bezos and Microsoft cofounder Bill Gates. After Gorham’s neurologist recommended that he try it, he became one of the first volunteers to have an 8mm stent, laced with small electrodes, implanted into his jugular vein and guided by a surgeon into a blood vessel near the part of his brain that controls movement.
After arriving at their destination, these tiny sensors can detect neural activity. They relay these messages through a small receiver implanted under the skin to a computer, which then translates the information into words. This minimally invasive surgery takes a day and is painless, according to Gorham. Recovery time is typically short, about two days.
When a paralyzed patient thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts.
When a paralyzed patient such as Gorham thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts. This pattern is detected by the Stentrode and relayed to a computer that learns to associate this pattern with the patient’s physical movements. The computer recognizes thoughts about kicking, making a fist and other movements as signals for clicking a mouse or pushing certain letters on a keyboard. An additional eye-tracking device controls the movement of the computer cursor.
The process works on a letter by letter basis. That’s why longer and more nuanced responses often involve some trial and error. “I have been using this for about two years, and I enjoy the sessions,” Gorham typed during our chat session. Zafar Faraz, field clinical engineer at Synchron, sat next to Gorham, providing help when required. Gorham had suffered without internet access, but now he looks forward to surfing the web and playing video games.

Gorham, age 63, has been enjoying Stentrode sessions for about two years.
Rodeny Dekker
The BCI revolution
In the summer of 2021, Synchron became the first company to receive the FDA’s Investigational Device Exemption, which allows research trials on the Stentrode in human patients. This past summer, the company, together with scientists from Icahn School of Medicine at Mount Sinai and the Neurology and Neurosurgery Department at Utrecht University, published a paper offering a framework for how to develop BCIs for patients with severe paralysis – those who can't use their upper limbs to type or use digital devices.
Three months ago, Synchron announced the enrollment of six patients in a study called COMMAND based in the U.S. The company will seek approval next year from the FDA to make the Stentrode available for sale commercially. Meanwhile, other companies are making progress in the field of BCIs. In August, Neuralink announced a $280 million financing round, the biggest fundraiser yet in the field. Last December, Synchron announced a $75 million financing round. “One thing I can promise you, in five years from now, we’re not going to be where we are today. We're going to be in a very different place,” says Elad I. Levy, professor of neurosurgery and radiology at State University of New York in Buffalo.
The risk of hacking exists, always. Cybercriminals, for example, might steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices while extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“The prospect of bestowing individuals with paralysis a renewed avenue for communication and motor functionality is a step forward in neurotech,” says Hayley Nelson, a neuroscientist and founder of The Academy of Cognitive and Behavioral Neuroscience. “It is an exciting breakthrough in a world of devastating, scary diseases,” says Neil McArthur, a professor of philosophy and director of the Centre for Professional and Applied Ethics at the University of Manitoba. “To connect with the world when you are trapped inside your body is incredible.”
While the benefits for the paraplegic community are promising, the Stentrode’s long-term effectiveness and overall impact needs more research on safety. “Potential risks like inflammation, damage to neural tissue, or unexpected shifts in synaptic transmission due to the implant warrant thorough exploration,” Nelson says.
There are also concens about data privacy concerns and the policies of companies to safeguard information processed through BCIs. “Often, Big Tech is ahead of the regulators because the latter didn’t envisage such a turn of events...and companies take advantage of the lack of legal framework to push forward,” McArthur says. Hacking is another risk. Cybercriminals could steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices. Extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“We have to protect patient identity, patient safety and patient integrity,” Levy says. “In the same way that we protect our phones or computers from hackers, we have to stay ahead with anti-hacking software.” Even so, Levy thinks the anticipated benefits for the quadriplegic community outweigh the potential risks. “We are on the precipice of an amazing technology. In the future, we would be able to connect patients to peripheral devices that enhance their quality of life.”
In the near future, the Stentrode could enable patients to use the Stentrode to activate their wheelchairs, iPods or voice modulators. Synchron's focus is on using its BCI to help patients with significant mobility restrictions—not to enhance the lives of healthy people without any illnesses. Levy says we are not prepared for the implications of endowing people with superpowers.
I wondered what Gorham thought about that. “Pardon my question, but do you feel like you have sort of transcended human nature, being the first in a big line of cybernetic people doing marvelous things with their mind only?” was my last question to Gorham.
A slight smile formed on his lips. In less than a minute, he typed: “I do a little.”
Leading XPRIZE Healthspan and Beating Negativity with Dr. Peter Diamandis
XPRIZE founder and chairman Peter Diamandis launches XPRIZE Healthspan at an event on November 29.
A new competition by the XPRIZE Foundation is offering $101 million to researchers who discover therapies that give a boost to people aged 65-80 so their bodies perform more like when they were middle-aged.
For today’s podcast episode, I talked with Dr. Peter Diamandis, XPRIZE’s founder and executive chairman. Under Peter’s leadership, XPRIZE has launched 27 previous competitions with over $300 million in prize purses. The latest contest aims to enhance healthspan, or the period of life when older people can play with their grandkids without any restriction, disability or disease. Such breakthroughs could help prevent chronic diseases that are closely linked to aging. These illnesses are costly to manage and threaten to overwhelm the healthcare system, as the number of Americans over age 65 is rising fast.
In this competition, called XPRIZE Healthspan, multiple awards are available, depending on what’s achieved, with support from the nonprofit Hevolution Foundation and Chip Wilson, the founder of Lululemon and nonprofit SOLVE FSHD. The biggest prize, $81 million, is for improvements in cognition, muscle and immunity by 20 years. An improvement of 15 years will net $71 million, and 10 years will net $61 million.
In our conversation for this episode, Peter talks about his plans for XPRIZE Healthspan and why exponential technologies make the current era - even with all of its challenges - the most exciting time in human history. We discuss the best mental outlook that supports a person in becoming truly innovative, as well as the downsides of too much risk aversion. We talk about how to overcome the negativity bias in ourselves and in mainstream media, how Peter has shifted his own mindset to become more positive over the years, how to inspire a culture of innovation, Peter’s personal recommendations for lifestyle strategies to live longer and healthier, the innovations we can expect in various fields by 2030, the future of education and the importance of democratizing tech and innovation.
In addition to Peter’s pioneering leadership of XPRIZE, he is also the Executive Founder of Singularity University. In 2014, he was named by Fortune as one of the “World’s 50 Greatest Leaders.” As an entrepreneur, he’s started over 25 companies in the areas of health-tech, space, venture capital and education. He’s Co-founder and Vice-Chairman of two public companies, Celularity and Vaxxinity, plus being Co-founder & Chairman of Fountain Life, a fully-integrated platform delivering predictive, preventative, personalized and data-driven health. He also serves as Co-founder of BOLD Capital Partners, a venture fund with a half-billion dollars under management being invested in exponential technologies and longevity companies. Peter is a New York Times Bestselling author of four books, noted during our conversation and in the show notes of this episode. He has degrees in molecular genetics and aerospace engineering from MIT and holds an M.D. from Harvard Medical School.
Show links
- Peter Diamandis bio
- New XPRIZE Healthspan
- Peter Diamandis books
- 27 XPRIZE competitions and counting
- Life Force by Peter Diamandis and Tony Robbins
- Peter Diamandis Twitter
- Longevity Insider newsletter – AI identifies the news
- Peter Diamandis Longevity Handbook
- Hevolution funding for longevity

XPRIZE Founder Peter Diamandis speaks with Mehmoud Khan, CEO of Hevolution Foundation, at the launch of XPRIZE Healthspan.
Hevolution Foundation

