How Bacteria-Killing Viruses May Save Us From Antibiotic Resistance
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
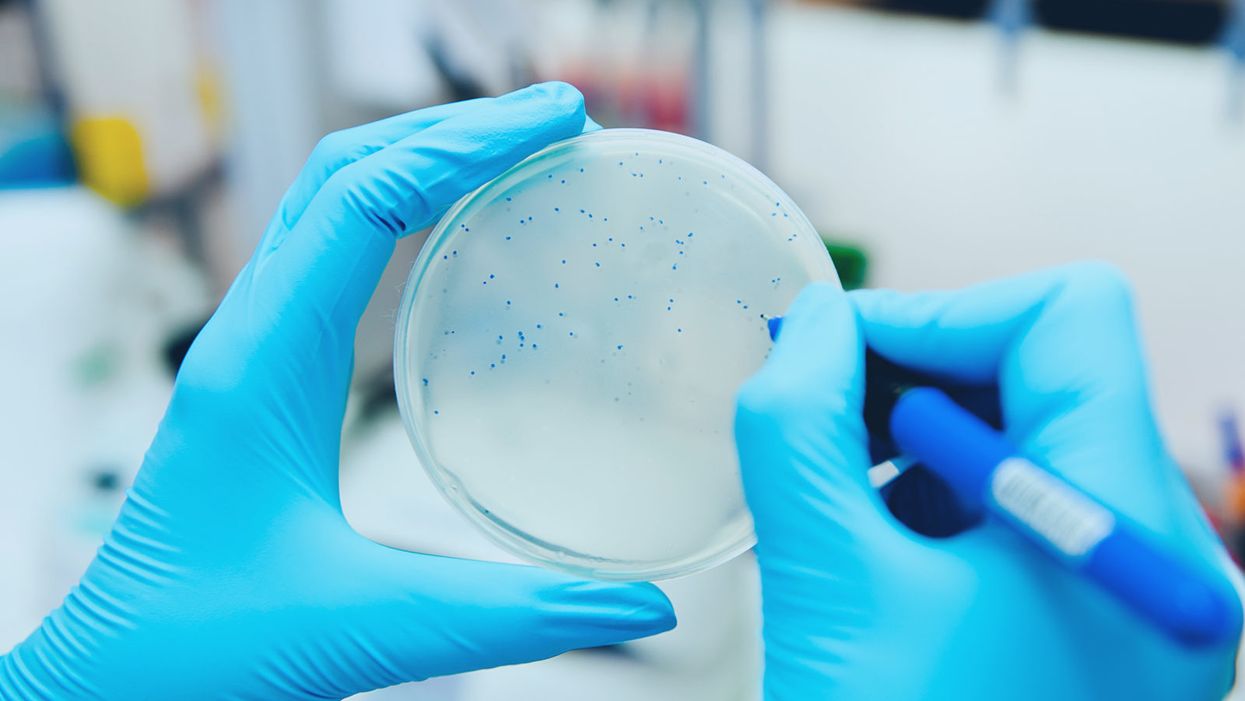
Hand-counting bacteriophage plaques during a titer test.
In my hometown of Pittsburgh, it is not uncommon to read about cutting-edge medical breakthroughs, because Pittsburgh is the home of many innovations in medical science, from the polio vaccine to pioneering organ transplantation. However, medical headlines from Pittsburgh last November weren't heralding a new discovery for once. They were carrying a plea—for a virus.
Phages are weapons of bacterial destruction, but despite recognition of their therapeutic potential for over 100 years, there are zero phage products commercially available to medicine in the United States.
Specifically, a bacteria-killing virus that could attack and control a certain highly drug-resistant bacterial infection ravaging the newly transplanted lungs of a 25-year-old woman named Mallory Smith. The culprit bacteria, Burkholderia cepacia, is a notoriously vicious bacterium that preys on patients with cystic fibrosis who, throughout their life, are exposed to course after course of antibiotics, often fostering a population of highly resistant bacteria that can become too formidable for modern medicine to combat.
What Smith and her physicians desperately needed was a tool that would move beyond failed courses of antibiotics. What they sought was called a bacteriophage. These are naturally occurring ubiquitous viruses that target not humans, but bacteria. The world literally teems with "phages" and one cannot take a bite or drink of anything without encountering them. These weapons of bacterial destruction are exquisitely evolved to target bacteria and, as such, are not harmful to humans. However, despite recognition of their therapeutic potential for over 100 years, there are zero bacteriophage products commercially available to medicine in the United States, at a time when antibiotic resistance is arguably our most pressing public health crisis. Just this week, a new study was published in the Proceedings of the National Academy of Sciences detailing the global scope of the problem.
Why Were These Promising Tools Forgotten?
Phages weren't always relegated to this status. In fact, in the early 20th century phages could be found on American drug store shelves and were used for a variety of ailments. However, the path-breaking discovery and development of antimicrobials agents such as the sulfa drugs and, later the antibiotic penicillin, supplanted the world of phage therapeutics in the United States and many other places.
Fortunately, phage therapy never fully disappeared, and research and clinical use continued in Eastern European nations such as Georgia and Poland.
The antibiotic age revolutionized medicine in a way that arguably no other innovation has. Not only did antibiotics tame many once-deadly infectious diseases, but they made much of modern medicine – from cancer chemotherapy to organ transplantation to joint replacement – possible. Antibiotics, unlike the exquisitely evolved bacteriophage, possessed a broader spectrum of activity and were active against a range of bacteria. This non-specificity facilitated antibiotic use without the need for a specific diagnosis. A physician does not need to know the specific bacterial genus and species causing, for example, a skin infection or pneumonia, but can select an antibiotic that covers the likely culprits and use it empirically, fully expecting the infection to be controlled. Unfortunately, this non-specificity engendered the overuse of antibiotics whose consequences we are now suffering. A bacteriophage, on the other hand, will work against one specific bacterial species and is evolved for just that role.
Phages to the Rescue
As the march of antibiotic resistance has predictably continued since the dawn of the antibiotic age, the prospect of resurrecting phage therapy has been increasingly viewed as one solution. Fortunately, phage therapy never fully disappeared, and research and clinical use continued in Eastern European nations such as Georgia and Poland. However, much of that experience has remained opaque to the medical community at large and questions about dosage, toxicity, efficacy, and method of delivery left many questions without full answers.
Though real questions remained regarding phage use, dire circumstances of prolific antibiotic resistance necessitated their use in the U.S. in two prominent instances involving life-threatening infections. The first case involved an Acinetobacter baumanii infection of the pancreas in a San Diego man in which phages were administered intravenously in 2016. The other case, also in 2016, involved the instillation of phages, fished out of a pond, into the chest cavity of man with a Pseudmonas aeruginosa infection of a prosthetic graft of the aorta. Both cases were successful and were what fueled the Pittsburgh-based plea for Burkholderia phages.
The phages you begin with may not be the ones you end up with, as Darwinian evolutionary pressures will alter the phage in order to keep up with the ongoing evolution of its bacterial target.
How Phages Differ from Other Medical Products
It might seem surprising that in light of the urgent need for new treatments for drug-resistant infections, the pharmaceutical armamentarium is not teeming with phages like a backyard pond. However, phages have been difficult to fit into the current regulatory framework that operates in most developed countries such as the U.S. because of their unique characteristics.
Phages are not one homogenous product like a tablet of penicillin, but a cocktail of viruses that change and evolve as they replicate. The phages you begin with may not be the ones you end up with, as Darwinian evolutionary pressures will alter the phage in order to keep up with the ongoing evolution of its bacterial target. The cocktail may not just contain one specific phage, but a range of phages that all target some specific bacteria in order to increase efficacy. These phage cocktails might also need adjusting to keep pace with bacterial resistance. Additionally, the concentration of phage in a human body after administration is not so easy to predict as phage numbers will rise and fall based on the number of target bacteria that are present.
All of these characteristics make phages very unique when viewed through a regulatory lens, and necessitate the creation of new methods to evaluate them, given that regulatory approval is required. Using phages in the U.S. now requires FDA permission through an investigational new drug application, which can be expedited during an emergency situation. FDA scientists are actively involved in understanding the best means to evaluate bacteriophage therapy and several companies are in early-stage development, though no major clinical trials in the U.S. are currently underway.
One FDA-approved application of phages has seen them used on food products at delis and even in slaughterhouses to diminish the quantity of bacteria on certain meat products.
Would That Humans Were As Lucky As Bologna
Because of the regulatory difficulties with human-use approval, some phage companies have taken another route to develop phage products: food safety. Food safety is a major public health endeavor, and keeping food that people consume safe from E.coli, Listeria, and Salmonella, for example, are rightfully major priorities of industry. One FDA-approved application of phages has seen them used on food products at delis and even in slaughterhouses to diminish the quantity of bacteria on certain meat products.
This use, unlike that for human therapeutic purposes, has found success with regulators: phages, not surprisingly, have been granted the "generally regarded as safe (GRAS)" designation.
A Phage Directory
Tragically Mallory Smith succumbed to her infection despite getting a dose of phages culled from sludge in the Philippines and Fiji. However, her death and last-minute crusade to obtain phages has prompted the call for a phage directory. This directory could catalog the various phages being studied and the particular bacteria they target. Such a searchable index will facilitate the rapid identification and – hopefully – delivery of phages to patients.
If phage therapy is to move from a last-ditch emergency measure to a routine tool for infectious disease physicians, it will be essential that the hurdles they face are eliminated.
Moving Beyond Antibiotics
As we move increasingly toward a post-antibiotic age in infectious disease, moving outside of the traditional paradigm of broad-spectrum antibiotics to non-traditional therapeutics such as bacteriophages and other novel products will become increasingly necessary. Already, clinical trials are underway in various populations, including a major trial in European burn patients.
It is important to understand that there are important scientific and therapeutic questions regarding dose, route of administration and other related questions that need to be addressed before phage use becomes more routine, and it is only through clinical trials conducted with the hope of eventual commercialization that these answers will be found. If phage therapy is to move from a last-ditch emergency measure to a routine tool for infectious disease physicians, it will be essential that the hurdles they face are eliminated.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained

Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

