How Can We Decide If a Biomedical Advance Is Ethical?

A newborn in the maternity ward. (© rufous/Fotolia)
"All fixed, fast-frozen relations, with their train of ancient and venerable prejudices and opinions, are swept away, all new-formed ones become antiquated before they can ossify. All that is solid melts into air, all that is holy is profaned…"
On July 25, 1978, Louise Brown was born in Oldham, England, the first human born through in vitro fertilization, through the work of Patrick Steptoe, a gynecologist, and Robert Edwards, a physiologist. Her birth was greeted with strong (though not universal) expressions of ethical dismay. Yet in 2016, the latest year for which we have data, nearly two percent of the babies born in the United States – and around the same percentage throughout the developed world – were the result of IVF. Few, if any, think of these children as unnatural, monsters, or freaks or of their parents as anything other than fortunate.
How should we view Dr. He today, knowing that the world's eventual verdict on the ethics of biomedical technologies often changes?
On November 25, 2018, news broke that Chinese scientist, Dr. He Jiankui, claimed to have edited the genomes of embryos, two of whom had recently become the new babies, Lulu and Nana. The response was immediate and overwhelmingly negative.
Times change. So do views. How will Dr. He be viewed in 40 years? And, more importantly, how should we view him today, knowing that the world's eventual verdict on the ethics of biomedical technologies often changes? And when what biomedicine can do changes with vertiginous frequency?
How to determine what is and isn't ethical is above my pay grade. I'm a simple law professor – I can't claim any deeper insight into how to live a moral life than the millennia of religious leaders, philosophers, ethicists, and ordinary people trying to do the right thing. But I can point out some ways to think about these questions that may be helpful.
First, consider two different kinds of ethical commands. Some are quite specific – "thou shalt not kill," for example. Others are more general – two of them are "do unto others as you would have done to you" or "seek the greatest good for the greatest number."
Biomedicine in the last two centuries has often surprised us with new possibilities, situations that cultures, religions, and bodies of ethical thought had not previously had to consider, from vaccination to anesthesia for women in labor to genome editing. Sometimes these possibilities will violate important and deeply accepted precepts for a group or a person. The rise of blood transfusions around World War I created new problems for Jehovah's Witnesses, who believe that the Bible prohibits ingesting blood. The 20th century developments of artificial insemination and IVF both ran afoul of Catholic doctrine prohibiting methods other than "traditional" marital intercourse for conceiving children. If you subscribe to an ethical or moral code that contains prohibitions that modern biomedicine violates, the issue for you is stark – adhere to those beliefs or renounce them.
If the harms seem to outweigh the benefits, it's easy to conclude "this is worrisome."
But many biomedical changes violate no clear moral teachings. Is it ethical or not to edit the DNA of embryos? Not surprisingly, the sacred texts of various religions – few of which were created after, at the latest, the early 19th century, say nothing specific about this. There may be hints, precedents, leanings that could argue one way or another, but no "commandments." In that case, I recommend, at least as a starting point, asking "what are the likely consequences of these actions?"
Will people be, on balance, harmed or helped by them? "Consequentialist" approaches, of various types, are a vast branch of ethical theories. Personally I find a completely consequentialist approach unacceptable – I could not accept, for example, torturing an innocent child even in order to save many lives. But, in the absence of a clear rule, looking at the consequences is a great place to start. If the harms seem to outweigh the benefits, it's easy to conclude "this is worrisome."
Let's use that starting place to look at a few bioethical issues. IVF, for example, once proven (relatively) safe seems to harm no one and to help many, notably the more than 8 million children worldwide born through IVF since 1978 – and their 16 million parents. On the other hand, giving unknowing, and unconsenting, intellectually disabled children hepatitis A harmed them, for an uncertain gain for science. And freezing the heads of the dead seems unlikely to harm anyone alive (except financially) but it also seems almost certain not to benefit anyone. (Those frozen dead heads are not coming back to life.)
Now let's look at two different kinds of biomedical advances. Some are controversial just because they are new; others are controversial because they cut close to the bone – whether or not they violate pre-established ethical or moral norms, they clearly relate to them.
Consider anesthesia during childbirth. When first used, it was controversial. After all, said critics, in Genesis, the Bible says God told Eve, "I will greatly multiply Your pain in childbirth, In pain you will bring forth children." But it did not clearly prohibit pain relief and from the advent of ether on, anesthesia has been common, though not universal, in childbirth in western societies. The pre-existing ethical precepts were not clear and the consequences weighed heavily in favor of anesthesia. Similarly, vaccination seems to violate no deep moral principle. It was, and for some people, still is just strange, and unnatural. The same was true of IVF initially. Opposition to all of these has faded with time and familiarity. It has not disappeared – some people continue to find moral or philosophical problems with "unnatural" childbirth, vaccination, and IVF – but far fewer.
On the other hand, human embryonic stem cell research touches deeper issues. Human embryos are destroyed to make those stem cells. Reasonable people disagree on the moral status of the human embryo, and the moral weight of its destruction, but it does at least bring into play clear and broadly accepted moral precepts, such as "Thou shalt not kill." So, at the far side of an individual's time, does euthanasia. More exposure to, and familiarity with, these practices will not necessarily lead to broad acceptance as the objections involve more than novelty.
The first is "what would I do?" The second – what should my government, culture, religion allow or forbid?
Finally, all this ethical analysis must work at two levels. The first is "what would I do?" The second – what should my government, culture, religion allow or forbid? There are many things I would not do that I don't think should be banned – because I think other people may reasonably have different views from mine. I would not get cosmetic surgery, but I would not ban it – and will try not to think ill of those who choose it
So, how should we assess the ethics of new biomedical procedures when we know that society's views may change? More specifically, what should we think of He Jiankui's experiment with human babies?
First, look to see whether the procedure in question violates, at least fairly clearly, some rule in your ethical or moral code. If so, your choice may not be difficult. But if the procedure is unmentioned in your moral code, probably because it was inconceivable to the code's creators, examine the consequences of the act.
If the procedure is just novel, and not something that touches on important moral concerns, looking at the likely consequences may be enough for your ethical analysis –though it is always worth remembering that predicting consequences perfectly is impossible and predicting them well is never certain. If it does touch on morally significant issues, you need to think those issues through. The consequences may be important to your conclusions but they may not be determinative.
And, then, if you conclude that it is not ethical from your perspective, you need to take yet another step and consider whether it should be banned for people who do not share your perspective. Sometimes the answer will be yes – that psychopaths may not view murder as immoral does not mean we have to let them kill – but sometimes it will be no.
What does this say about He Jiankui's experiment? I have no qualms in condemning it, unequivocally. The potential risks to the babies grossly outweighed any benefits to them, and to science. And his secret work, against a near universal scientific consensus, privileged his own ethical conclusions without giving anyone else a vote, or even a voice.
But if, in ten or twenty years, genome editing of human embryos is shown to be safe (enough) and it is proposed to be used for good reasons – say, to relieve human suffering that could not be treated in other good ways – and with good consents from those directly involved as well as from the relevant society and government – my answer might well change. Yours may not. Bioethics is a process for approaching questions; it is not a set of universal answers.
This article opened with a quotation from the 1848 Communist Manifesto, referring to the dizzying pace of change from industrialization and modernity. You don't need to be a Marxist to appreciate that sentiment. Change – especially in the biosciences – keeps accelerating. How should we assess the ethics of new biotechnologies? The best we can, with what we know, at the time we inhabit. And, in the face of vast uncertainty, with humility.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
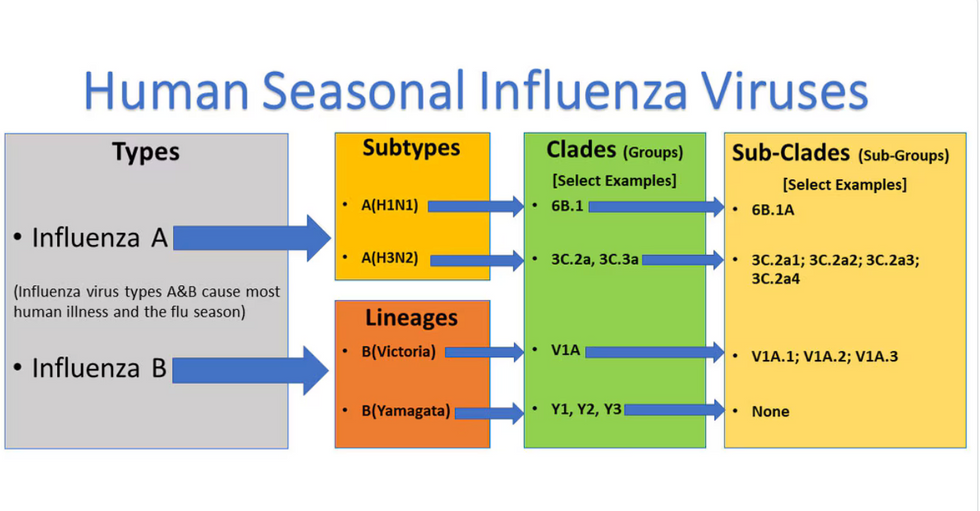
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

