Don't Panic Over Waning Antibodies. Here's Why.
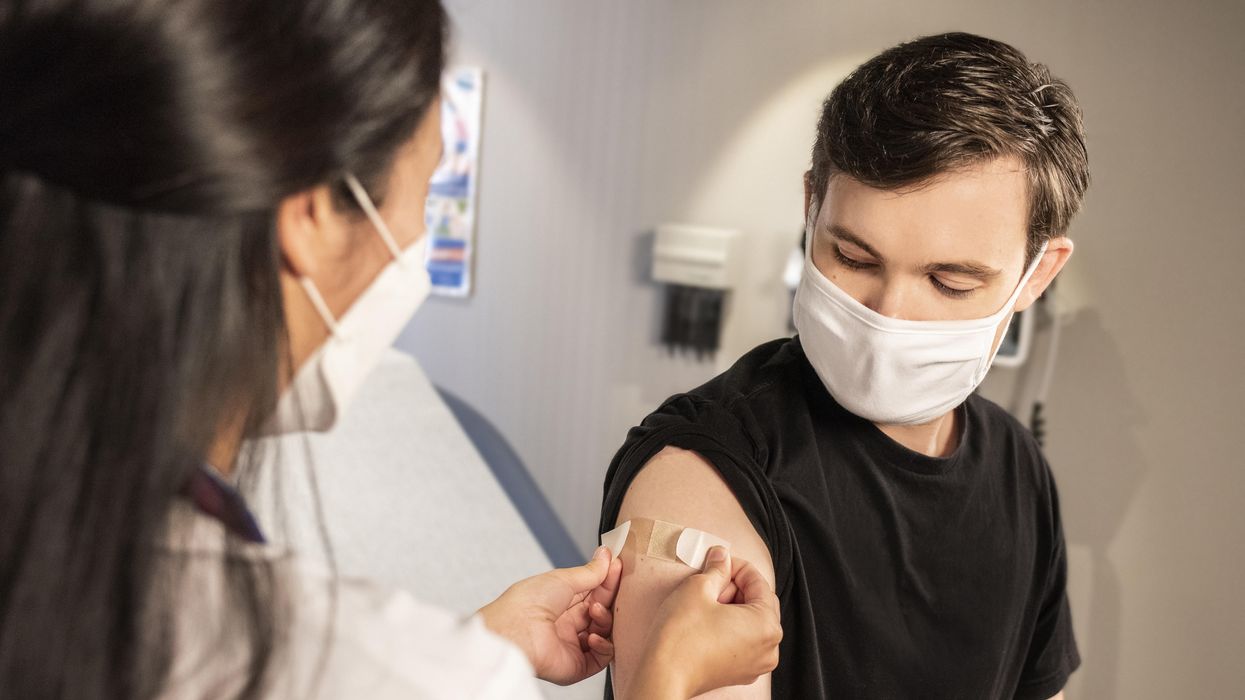
A health care worker places a bandaid on the arm of a man who has just been vaccinated.
Since the Delta variant became predominant in the United States on July 7, both scientists and the media alike have been full of mixed messages ("breakthrough infections rare"; "breakthrough infections common"; "vaccines still work"; "vaccines losing their effectiveness") but – if we remember our infectious diseases history- one thing remains clear: immunity is the only way to get through a pandemic.
What Happened in the Past
The 1918 influenza pandemic was far the deadliest respiratory virus pandemic recorded in recent human history with over 50 million deaths (maybe even 100 million deaths, or 3% of the world's population) worldwide. Although they used some of the same measures we are using now (masks, distancing, event closures, as neither testing nor a vaccine existed back then), the deaths slowed only after enough of the population had either acquired immunity through natural infection or died. Indeed, the first influenza vaccine was not developed until 1942, more than 20 years later. As judged by the amount of suffering and death from 1918 influenza (and the deadly Delta surge in India in spring 2021), natural immunity is obviously a terrible way to get through a pandemic.
Similarly, measles was a highly transmissible respiratory virus that led to high levels of immunity among adults who were invariably exposed as children. However, measles led to deaths each year among the nonimmune until a vaccine was developed in 1963, largely restricting current measles outbreaks in the U.S. now to populations who decline to vaccinate. Smallpox also led to high levels of immunity through natural infection, which was often fatal. That's why unleashing smallpox on a largely nonimmune population in the New World was so deadly. Only an effective vaccine – and its administration worldwide, including among populations who declined smallpox vaccine at first via mandates – could control and then eventually eradicate smallpox from Earth.
Fully vaccinated people are already now able to generate some antibodies against all the variants we know of to date, thanks to their bank of memory B cells.
The Delta variant is extremely transmissible, making it unlikely we will ever eliminate or eradicate SARS-CoV-2. Even Australia, which had tried to maintain a COVID-zero nation with masks, distancing, lockdowns, testing and contact tracing before and during the vaccines, ended a strategy aimed at eliminating COVID-19 this week. But, luckily, since highly effective and safe vaccines were developed for COVID-19 less than a year after its advent on a nonimmune population and since vaccines are retaining their effectiveness against severe disease, we have a safe way out of the misery of this pandemic: more and more immunity. "Defanging" SARS-CoV-2 and stripping it of its ability to cause severe disease through immunity will relegate it to the fate of the other four circulating cold-causing coronaviruses, an inconvenience but not a world-stopper.
Immunity Is More Than Antibodies
When we say immunity, we have to be clear that we are talking about cellular immunity and immune memory, not only antibodies. This is a key point. Neutralizing antibodies, which prevent the virus from entering our cells, are generated by the vaccines. But those antibodies will necessarily wane over time since we cannot keep antibodies from every infection and vaccine we have ever seen in the bloodstream (or our blood would be thick as paste!). Vaccines with shorter intervals between doses (like Pfizer vaccines given 3 weeks apart) are likely to have their antibodies wane sooner than vaccines with longer intervals between doses (like Moderna), making mild symptomatic breakthroughs less likely with the Moderna vaccine than the Pfizer during our Delta surge, as a recent Mayo Clinic study showed.
Luckily, the vaccines generate B cells that get relegated to our memory banks and these memory B cells are able to produce high levels of antibodies to fight the virus if they see it again. Amazingly, these memory B cells will actually produce antibodies adapted against the COVID variants if they see a variant in the future, rather than the original antibodies directed against the ancestral strain. This is because memory B cells serve as a blueprint to make antibodies, like the blueprint of a house. If a house needs an extra column (or antibodies need to evolve to work against variants), the blueprint will oblige just as memory B cells will!
One problem with giving a 3rd dose of our current vaccines is that those antibodies won't be adapted towards the variants. Fully vaccinated people are already now able to generate some antibodies against all the variants we know of to date, thanks to their bank of memory B cells. In other words, no variant has evolved to date that completely evades our vaccines. Memory B cells, once generated by either natural infection or vaccination, should be long-lasting.
If memory B cells are formed by a vaccine, they should be as long-lasting as those from natural infection per various human studies. A 2008 Nature study found that survivors of the 1918 influenza pandemic were able to produce antibodies from memory B cells when exposed to the same influenza strain nine decades later. Of note, mild infections (such as the common cold from the cold-causing coronaviruses called 229E, NL63, OC43, and HKU1) may not reliably produce memory B cell immunity like more severe infections caused by SARS-CoV-2.
Right about now, you may be worrying about a super-variant that might yet emerge to evade all our hard-won immune responses. But most immunologists do not think this is very realistic because of T cells. How are T cells different from B Cells? While B cells are like the memory banks to produce antibodies when needed (helped by T cells), T cells will specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly. We likely have T cells to thank for the vaccine's incredible durability in protecting us against severe disease. Data from La Jolla Immunology Institute and UCSF show that the T cell response from the Pfizer vaccine is strong across all the variants.
Think of your spike protein as being comprised of 100 houses with a T cell there to cover each house (to protect you against severe disease). The variants have around 13 mutations along the spike protein so 13 of those T cells won't work, but there are over 80 T cells remaining to protect your "houses" or your body against severe disease.
Although these are theoretical numbers and we don't know exactly the number of T cell antigens (or "epitopes") across the spike protein, one review showed 1400 across the whole virus, with many of those in the spike protein. Another study showed that there were 87 epitopes across the spike protein to which T cells respond, and mutations in one of the variants (beta) took those down to 75. The virus cannot mutate indefinitely in its spike protein and still retain function. This is why it is unlikely we will get a variant that will evade the in-breadth, robust response of our T cells.
Where We Go From Here
So, what does this mean for getting through this pandemic? Immunity and more immunity. For those of us who are vaccinated, if we get exposed to the Delta variant, it will boost our immune response although the memory B cells might take 3-5 days to make new antibodies, which can leave us susceptible to a mild breakthrough infection. That's part of the reason the CDC put back masks for the vaccinated in late July. For those who are unvaccinated, immunity will be gained from Delta but often through terrible ways like severe disease.
The way for the U.S. to determine the need for 3rd shots among those who are not obviously immunocompromised, given the amazing immune memory generated by the vaccines among immunocompetent individuals, is to analyze the cases of the ~6000 individuals who have had severe breakthrough infections among the 171 million Americans fully vaccinated. Define their co-morbidities and age ranges, and boost those susceptible to severe infections (examples could include older people, those with co-morbidities, health care workers, and residents of long-term care facilities). This is an approach likely to be taken by the CDC's Advisory Committee on Immunization Practices.
If immunity is the only way to get through the pandemic and if variants are caused mostly by large populations being unvaccinated, there is not only a moral and ethical imperative but a practical imperative to vaccinate the world in order to keep us all safe. Immunocompetent Americans can boost their antibodies, which may enhance their ability to avoid mild breakthrough infections, but the initial shots still work well against the most important outcomes: hospitalizations and deaths.
There has been no randomized, controlled trial to assess whether three shots vs. two shots meaningfully improve those outcomes. While we ought to trust immune memory to get the immunocompetent in the United States through, we can hasten the end of this pandemic by providing surplus vaccines to poor countries to combat severe disease. Doing so would not only revitalize the role of the U.S. as a global health leader – it would save countless lives.
Avalanche rescue dogs train to find and dig out people buried in snow slides
Two-and-a-half year-old Huckleberry, a blue merle Australian shepherd, pulls hard at her leash; her yelps can be heard by skiers and boarders high above on the chairlift that carries them over the ski patrol hut to the top of the mountain. Huckleberry is an avalanche rescue dog — or avy dog, for short. She lives and works with her owner and handler, a ski patroller at Breckenridge Ski Resort in Colorado. As she watches the trainer play a game of hide-and-seek with six-month-old Lume, a golden retriever and avy dog-in-training, Huckleberry continues to strain on her leash; she loves the game. Hide-and-seek is one of the key training methods for teaching avy dogs the rescue skills they need to find someone caught in an avalanche — skier, snowmobiler, hiker, climber.
Lume’s owner waves a T-shirt in front of the puppy. While another patroller holds him back, Lume’s owner runs away and hides. About a minute later — after a lot of barking — Lume is released and commanded to “search.” He springs free, running around the hut to find his owner who reacts with a great amount of excitement and fanfare. Lume’s scent training will continue for the rest of the ski season (Breckenridge plans operating through May or as long as weather permits) and through the off-season. “We make this game progressively harder by not allowing the dog watch the victim run away,” explains Dave Leffler, Breckenridge's ski patroller and head of the avy dog program, who has owned, trained and raised many of them. Eventually, the trainers “dig an open hole in the snow to duck out of sight and gradually turn the hole into a cave where the dog has to dig to get the victim,” explains Leffler.
By the time he is three, Lume, like Huckleberry, will be a fully trained avy pup and will join seven other avy dogs on Breckenridge ski patrol team. Some of the team members, both human and canine, are also certified to work with Colorado Rapid Avalanche Deployment, a coordinated response team that works with the Summit County Sheriff’s office for avalanche emergencies outside of the ski slopes’ boundaries.
There have been 19 avalanche deaths in the U.S. this season, according to avalanche.org, which tracks slides; eight in Colorado. During the entirety of last season there were 17. Avalanche season runs from November through June, but avalanches can occur year-round.
High tech and high stakes
Complementing avy dogs’ ability to smell people buried in a slide, avalanche detection, rescue and recovery is becoming increasingly high tech. There are transceivers, signal locators, ground scanners and drones, which are considered “games changers” by many in avalanche rescue and recovery
For a person buried in an avalanche, the chance of survival plummets after 20 minutes, so every moment counts.
A drone can provide thermal imaging of objects caught in a slide; what looks like a rock from far away might be a human with a heat signature. Transceivers, also known as beacons, send a signal from an avalanche victim to a companion. Signal locators, like RECCO reflectors which are often sewn directly into gear, can echo back a radar signal sent by a detector; most ski resorts have RECCO detector units.
Research suggests that Ground Penetrating Radar (GPR), an electromagnetic tool used by geophysicists to pull images from inside the ground, could be used to locate an avalanche victim. A new study from the Department of Energy’s Sandia National Laboratories suggests that a computer program developed to pinpoint the source of a chemical or biological terrorist attack could also be used to find someone submerged in an avalanche. The search algorithm allows for small robots (described as cockroach-sized) to “swarm” a search area. Researchers say that this distributed optimization algorithm can help find avalanche victims four times faster than current search mechanisms. For a person buried in an avalanche, the chance of survival plummets after 20 minutes, so every moment counts.

An avy dog in training is picking up scent
Sarah McLear
While rescue gear has been evolving, predicting when a slab will fall remains an emerging science — kind of where weather forecasting science was in the 1980s. Avalanche forecasting still relies on documenting avalanches by going out and looking,” says Ethan Greene, director of the Colorado Avalanche Information Center (CAIC). “So if there's a big snowstorm, and as you might remember, most avalanches happened during snowstorms, we could have 10,000 avalanches that release and we document 50,” says Greene. “Avalanche forecasting is essentially pattern recognition,” he adds--and understanding the layering structure of snow.
However, determining where the hazards lie can be tricky. While a dense layer of snow over a softer, weaker layer may be a recipe for an avalanche, there’s so much variability in snowpack that no one formula can predict the trigger. Further, observing and measuring snow at a single point may not be representative of all nearby slopes. Finally, there’s not enough historical data to help avalanche scientists create better prediction models.
That, however, may be changing.
Last year, an international group of researchers created computer simulations of snow cover using 16 years of meteorological data to forecast avalanche hazards, publishing their research in Cold Regions Science and Technology. They believe their models, which categorize different kinds of avalanches, can support forecasting and determine whether the avalanche is natural (caused by temperature changes, wind, additional snowfall) or artificial (triggered by a human or animal).
With smell receptors ranging from 800 million for an average dog, to 4 billion for scent hounds, canines remain key to finding people caught in slides.
With data from two sites in British Columbia and one in Switzerland, researchers built computer simulations of five different avalanche types. “In terms of real time avalanche forecasting, this has potential to fill in a lot of data gaps, where we don't have field observations of what the snow looks like,” says Simon Horton, a postdoctoral fellow with the Simon Fraser University Centre for Natural Hazards Research and a forecaster with Avalanche Canada, who participated in the study. While complex models that simulate snowpack layers have been around for a few decades, they weren’t easy to apply until recently. “It's been difficult to find out how to apply that to actual decision-making and improving safety,” says Horton. If you can derive avalanche problem types from simulated snowpack properties, he says, you’ll learn “a lot about how you want to manage that risk.”
The five categories include “new snow,” which is unstable and slides down the slope, “wet snow,” when rain or heat makes it liquidly, as well as “wind-drifted snow,” “persistent weak layers” and “old snow.” “That's when there's some type of deeply buried weak layer in the snow that releases without any real change in the weather,” Horton explains. “These ones tend to cause the most accidents.” One step by a person on that structurally weak layer of snow will cause a slide. Horton is hopeful that computer simulations of avalanche types can be used by scientists in different snow climates to help predict hazard levels.
Greene is doubtful. “If you have six slopes that are lined up next to each other, and you're going to try to predict which one avalanches and the exact dimensions and what time, that's going to be really hard to do. And I think it's going to be a long time before we're able to do that,” says Greene.
What both researchers do agree on, though, is that what avalanche prediction really needs is better imagery through satellite detection. “Just being able to count the number of avalanches that are out there will have a huge impact on what we do,” Greene says. “[Satellites] will change what we do, dramatically.” In a 2022 paper, scientists at the University of Aberdeen in England used satellites to study two deadly Himalayan avalanches. The imaging helped them determine that sediment from a 2016 ice avalanche plus subsequent snow avalanches contributed to the 2021 avalanche that caused a flash flood, killing over 200 people. The researchers say that understanding the avalanches characteristics through satellite imagery can inform them how one such event increases the magnitude of another in the same area.

Avy dogs trainers hide in dug-out holes in the snow, teaching the dogs to find buried victims
Sarah McLear
Lifesaving combo: human tech and Mother Nature’s gear
Even as avalanche forecasting evolves, dogs with their built-in rescue mechanisms will remain invaluable. With smell receptors ranging from 800 million for an average dog, to 4 billion for scent hounds, canines remain key to finding people caught in slides. (Humans in comparison, have a meager 12 million.) A new study published in the Journal of Neuroscience revealed that in dogs smell and vision are connected in the brain, which has not been found in other animals. “They can detect the smell of their owner's fingerprints on a glass slide six weeks after they touched it,” says Nicholas Dodman, professor emeritus at Cummings School of Veterinary Medicine at Tufts University. “And they can track from a boat where a box filled with meat was buried in the water, 100 feet below,” says Dodman, who is also co-founder and president of the Center for Canine Behavior Studies.
Another recent study from Queens College in Belfast, United Kingdom, further confirms that dogs can smell when humans are stressed. They can also detect the smell of a person’s breath and the smell of the skin cells of a deceased person.
The emerging avalanche-predicting human-made tech and the incredible nature-made tech of dogs’ olfactory talents is the lifesaving “equipment” that Leffler believes in. Even when human-made technology develops further, it will be most efficient when used together with the millions of dogs’ smell receptors, Leffler believes. “It is a combination of technology and the avalanche dog that will always be effective in finding an avalanche victim.”
Living with someone changes your microbiome, new research shows
For the first time, research has shown that bacteria of the microbiome are transmitted between many individuals, not just infants and their mothers, in ways that can’t be explained by having the same diet or geography.
Some roommate frustration can be expected, whether it’s a sink piled high with crusty dishes or crumbs where a clean tabletop should be. Now, research suggests a less familiar issue: person-to-person transmission of shared bacterial strains in our gut and oral microbiomes. For the first time, the lab of Nicola Segata, a professor of genetics and computational biology at the University of Trento, located in Italy, has shown that bacteria of the microbiome are transmitted between many individuals, not just infants and their mothers, in ways that can’t be explained by their shared diet or geography.
It’s a finding with wide-ranging implications, yet frustratingly few predictable outcomes. Our microbiomes are an ever-growing and changing collection of helpful and harmful bacteria that we begin to accumulate the moment we’re born, but experts are still struggling to unravel why and how bacteria from one person’s gut or mouth become established in another person’s microbiome, as opposed to simply passing through.
“If we are looking at the overall species composition of the microbiome, then there is an effect of age of course, and many other factors,” Segata says. “But if we are looking at where our strains are coming from, 99 percent of them are only present in other people’s guts. They need to come from other guts.”
If we could better understand this process, we might be able to control and use it; perhaps hospital patients could avoid infections from other patients when their microbiome is depleted by antibiotics and their immune system is weakened, for example. But scientists are just beginning to link human microbiomes with various ailments. Growing evidence shows that our microbiomes steer our long-term health, impacting conditions like obesity, irritable bowel syndrome, type 2 diabetes, and cancer.
Previous work from Segata’s lab and others illuminated the ways bacteria are passed from mothers to infants during the first few months of life during vaginal birth, breastfeeding and other close contact. And scientists have long known that people in close proximity tend to share bacteria. But the factors related to that overlap, such as genetics and diet, were unclear, especially outside the mother-baby dyad.
“If we look at strain sharing between a mother and an infant at five years of age, for example, we cannot really tell which was due to transmission at birth and which is due to continued transmission because of contact,” Segata says. Experts hypothesized that they could be caused by bacterial similarities in the environment itself, genetics, or bacteria from shared foods that colonized the guts of people in close contact.
Strain sharing was highest in mother-child pairs, with 96 percent of them sharing strains, and only slightly lower in members of shared households, at 95 percent.
In Italy, researchers led by Mireia Valles-Colomer, including Segata, hoped to unravel this mystery. They compared data from 9,715 stool and saliva samples in 31 genomic datasets with existing metadata. Scientists zoomed in on variations in each bacterial strain down to the individual level. They examined not only mother-child pairs, but people living in the same household, adult twins, and people living in the same village in a level of detail that wasn’t possible before, due to its high cost and difficulties in retrieving data about interactions between individuals, Segata explained.
“This paper is, with high granularity, quantifying the percent sharing that you expect between different types of social interactions, controlling for things like genetics and diet,” Gibbons says. Strain sharing was highest in mother-child pairs, with 96 percent of them sharing strains, and only slightly lower in members of shared households, at 95 percent. And at least half of the mother-infant pairs shared 30 percent of their strains; the median was 12 percent among people in shared households. Yet, there was no sharing among eight percent of adult twins who lived separately, and 16 percent of people within villages who resided in different households. The results were published in Nature.
It’s not a regional phenomenon. Although the types of bacterial strains varied depending on whether people lived in western and eastern nations — datasets were drawn from 20 countries on five continents — the patterns of sharing were much the same. To establish these links, scientists focused on individual variations in shared bacterial strains, differences that create unique bacterial “fingerprints” in each person, while controlling for variables like diet, demonstrating that the bacteria had been transmitted between people and were not the result of environmental similarities.
The impact of this bacterial sharing isn’t clear, but shouldn’t be viewed with trepidation, according to Sean Gibbons, a microbiome scientist at the nonprofit Institute for Systems Biology.
“The vast majority of these bugs are actually either benign or beneficial to our health, and the fact that we're swapping and sharing them and that we can take someone else's strain and supplement or better diversify our own little garden is not necessarily a bad thing,” he says.
"There are hundreds of billions of dollars of investment capital moving into these microbiome therapeutic companies; bugs as drugs, so to speak,” says Sean Gibbons, a microbiome scientist at the Institute for Systems Biology.
Everyday habits like exercising and eating vegetables promote a healthy, balanced gut microbiome, which is linked to better metabolic and immune function, and fewer illnesses. While many people’s microbiomes contain bacteria like C. diff or E. coli, these bacteria don’t cause diseases in most cases because they’re present in low levels. But a microbiome that’s been wiped out by, say, antibiotics, may no longer keep these bacteria in check, allowing them to proliferate and make us sick.
“A big challenge in the microbiome field is being able to rationally predict whether, if you're exposed to a particular bug, it will stick in the context of your specific microbiome,” Gibbons says.
Gibbons predicts that explorations of microbe-based therapeutics will be “exploding” in the coming decades. “There are hundreds of billions of dollars of investment capital moving into these microbiome therapeutic companies; bugs as drugs, so to speak,” he says. Rather than taking a mass-marketed probiotic, a precise understanding of an individual’s microbiome could help target the introduction of just the right bacteria at just the right time to prevent or treat a particular illness.
Because the current study did not differentiate between different types of contact or relationships among household members sharing bacterial strains or determine the direction of transmission, Segata says his current project is examining children in daycare settings and tracking their microbiomes over time to understand the role genetics and everyday interactions play in the level of transmission that occurs.
This relatively newfound ability to trace bacterial variants to minute levels has unlocked the chance for scientists to untangle when and how bacteria leap from one microbiome to another. As researchers come to better understand the factors that permit a strain to establish itself within a microbiome, they could uncover new strategies to control these microbes, harnessing the makeup of each microbiome to help people to resist life-altering medical conditions.

