How One Doctor Single-Handedly Saved Countless Babies from Birth Defects

President John F. Kennedy gave Dr. Frances Oldham Kelsey the nation's highest federal civilian service award in 1962, saying she had "prevented a major tragedy of birth deformities."
In July 1956, a new drug hit the European market for the first time. The drug was called thalidomide – a sedative that was considered so safe it was available without a prescription.
Sedatives were in high demand in post-war Europe – but barbiturates, the most widely-used sedative at the time, caused overdoses and death when consumers took more than the recommended amount. Thalidomide, on the other hand, didn't appear to cause any side effects at all: Chemie Grünenthal, thalidomide's manufacturer, dosed laboratory rodents with over 600 times the normal dosage during clinical testing and had observed no evidence of toxicity.
The drug therefore was considered universally safe, and Grünenthal supplied thousands of doctors with samples to give to their patients. Doctors were encouraged to recommend thalidomide to their pregnant patients specifically because it was so safe, in order to relieve the nausea and insomnia associated with the first trimester of pregnancy.
By 1960, Thalidomide was being sold in countries throughout the world, and the United States was expected to soon follow suit. Dr. Frances Oldham Kelsey, a pharmacologist and physician, was hired by the Food and Drug Administration (FDA) in September of that year to review and approve drugs for the administration. Immediately, Kelsey was tasked with approving thalidomide for commercial use in the United States under the name Kevadon. Kelsey's approval was supposed to be a formality, since the drug was so widely used in other countries.
But Kelsey did something that few people expected – she paused. Rather than approving the drug offhand as she was expected to do, Kelsey asked the manufacturer – William S. Merrell Co., who was manufacturing thalidomide under license from Chemie Grünenthal – to supply her with more safety data, noting that Merrell's application for approval relied mostly on anecdotal testimony. Kelsey – along with her husband who worked as a pharmacologist at the National Institutes of Health (NIH) — was highly suspicious of a drug that had no lethal dose and no side effects. "It was just too positive," Kelsey said later. "This couldn't be the perfect drug with no risk."
At the same time, rumors were starting to swirl across Europe that thalidomide was not as safe as everyone had initially thought: Physicians were starting to notice an "unusual increase" in the birth of severely deformed babies, and they were beginning to suspect thalidomide as the cause. The babies, whose mothers had all taken thalidomide during pregnancy, were born with conditions like deafness, blindness, congenital heart problems, and even phocomelia, a malformation of the arms and legs. Doctors and midwives were also starting to notice a sharp rise in miscarriages and stillbirths among their patients as well.
Kelsey's skepticism was rewarded in November 1961 when thalidomide was yanked abruptly off the market, following a growing outcry that it was responsible for hundreds of stillbirths and deformities.
Kelsey had heard none of these rumors, but she did know from her post-doctoral research that adults could metabolize drugs differently than fetuses – in other words, a drug that was perfectly safe for adults could be detrimental to a patient's unborn child. Noting that thalidomide could cross the placental barrier, she asked for safety data, such as clinical trials, that showed specifically the drug was non-toxic for fetuses. Merrell supplied Kelsey with anecdotal data – in other words, accounts from patients who attested to the fact that they took thalidomide with no adverse effects – but she rejected it, needing stronger data: clinical studies with pregnant women included.
The drug company was annoyed at what they considered Kelsey's needless bureaucracy. After all, Germans were consuming around 1 million doses of thalidomide every day in 1960, with lots of anecdotal evidence that it was safe, even among pregnant women. As the holidays approached – the most lucrative time of year for sedative sales – Merrell executives started hounding Kelsey to approve thalidomide, even phoning her superior and paying her visits at work. But Kelsey was unmovable. Kelsey's skepticism was solidified in December 1960, when she read a letter published in the British Medical Journal from a physician. In the letter, the author warned that his long-term thalidomide patients were starting to report pain in their arms and legs.
"The burden of proof that the drug is safe … lies with the applicant," Kelsey wrote in a letter to Merrell executive Joseph F. Murray in May of 1961. Despite increasing pressure, Kelsey held fast to her insistence that more safety data – particularly for fetuses – was needed.
Kelsey's skepticism was rewarded in November 1961 when Chemie Grünenthal yanked thalidomide off the market overseas, following a growing outcry that it was responsible for hundreds of stillbirths and deformities. In early 1962, Merrell conceded that the drug's safety was unproven in fetuses and formally withdrew its application at the FDA.
Thanks to Kelsey, the United States was spared the effects of thalidomide – although countries like Europe and Canada were not so lucky. Thalidomide remained in people's homes under different names long after it was pulled from the market, and so women unfortunately continued to take thalidomide during their pregnancies, unaware of its effects. All told, thalidomide is thought to have caused around 10,000 birth defects and anywhere from 5,000 to 7,000 miscarriages. Many so-called "thalidomide babies" are now adults living with disabilities.

Niko von Glasow, born in 1960, is a German film director and producer who was born disabled due to the side effects of thalidomide.
Wikimedia Commons
Just two years after joining the FDA, Kelsey was presented with the President's Award for Distinguished Federal Civilian Service and was appointed as the head of the Investigational Drug Branch at the FDA. Not only did Kelsey save the U.S. public from the horrific effects of thalidomide, but she forever changed the way drugs were developed and approved for use in the United States: Drugs now need to not only be proven safe and effective, but adverse drug reactions need to be reported to the FDA and informed consent must be obtained by all participants before they volunteer for clinical trials. Today, the United States is safer because of Frances Kelsey's bravery.
The Friday Five: How to exercise for cancer prevention
How to exercise for cancer prevention. Plus, a device that brings relief to back pain, ingredients for reducing Alzheimer's risk, the world's oldest disease could make you young again, and more.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- How to exercise for cancer prevention
- A device that brings relief to back pain
- Ingredients for reducing Alzheimer's risk
- Is the world's oldest disease the fountain of youth?
- Scared of crossing bridges? Your phone can help
New approach to brain health is sparking memories
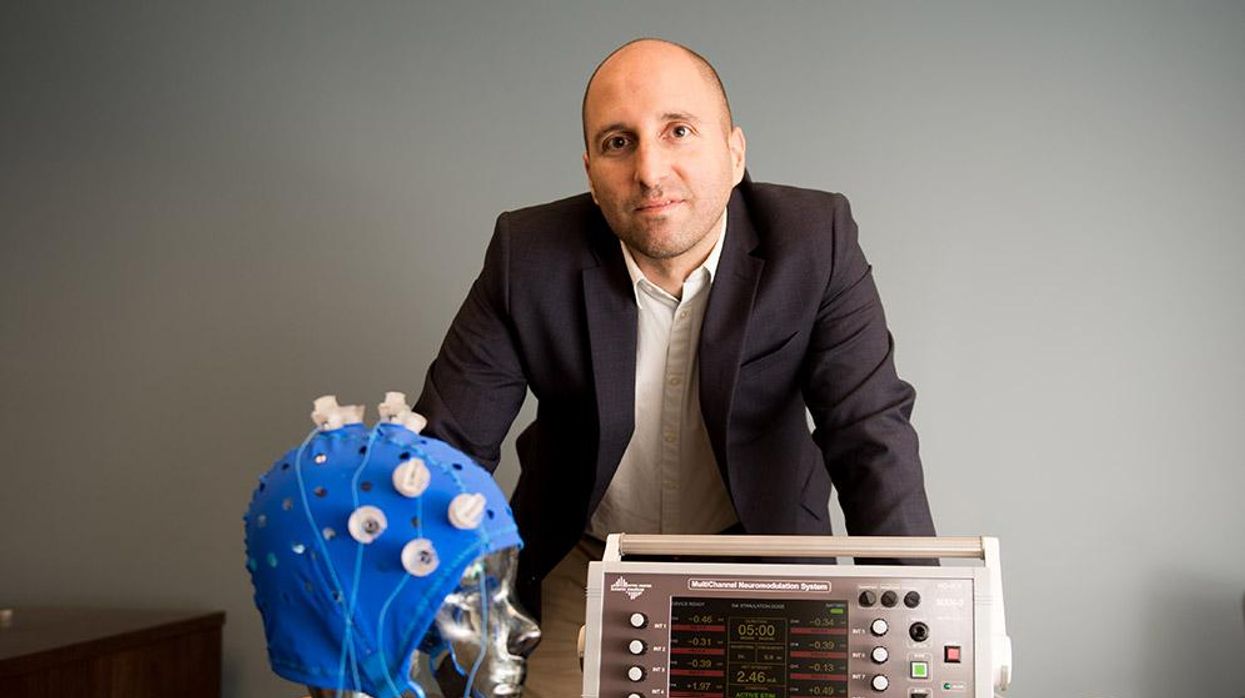
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”