Hyperbaric oxygen therapy could treat Long COVID, new study shows

Hyperbaric oxygen therapy has been used in the past to help people with traumatic brain injury, stroke and other conditions involving wounds to the brain. Now, researchers at Shamir Medical Center in Tel Aviv are studying how it could treat Long Covid.
Long COVID is not a single disease, it is a syndrome or cluster of symptoms that can arise from exposure to SARS-CoV-2, a virus that affects an unusually large number of different tissue types. That's because the ACE2 receptor it uses to enter cells is common throughout the body, and inflammation from the immune response fighting that infection can damage surrounding tissue.
One of the most widely shared groups of symptoms is fatigue and what has come to be called “brain fog,” a difficulty focusing and an amorphous feeling of slowed mental functioning and capacity. Researchers have tied these COVID-related symptoms to tissue damage in specific sections of the brain and actual shrinkage in its size.
When Shai Efrati, medical director of the Sagol Center for Hyperbaric Medicine and Research in Tel Aviv, first looked at functional magnetic resonance images (fMRIs) of patients with what is now called long COVID, he saw “micro infarcts along the brain.” It reminded him of similar lesions in other conditions he had treated with hyperbaric oxygen therapy (HBOT). “Once we saw that, we said, this is the type of wound we can treat. It doesn't matter if the primary cause is mechanical injury like TBI [traumatic brain injury] or stroke … we know how to oxidize them.”Efrati came to HBOT almost by accident. The physician had seen how it had helped heal diabetic ulcers and improved the lives of other patients, but he was busy with his own research. Then the director of his Tel Aviv hospital threatened to shut down the small HBOT chamber unless Efrati took on administrative responsibility for it. He reluctantly agreed, a decision that shifted the entire focus of his research.
“The main difference between wounds in the leg and wounds in the brain is that one is something we can see, it's tangible, and the wound in the brain is hidden,” says Efrati. With fMRIs, he can measure how a limited supply of oxygen in blood is shuttled around to fuel activity in various parts of the brain. Years of research have mapped how specific areas of the brain control activity ranging from thinking to moving. An fMRI captures the brain area as it’s activated by supplies of oxygen; lack of activity after the same stimuli suggests damage has occurred in that tissue. Suddenly, what was hidden became visible to researchers using fMRI. It helped to make a diagnosis and measure response to treatment.
HBOT is not a single thing but rather a tool, a process or approach with variations depending on the condition being treated. It aims to increase the amount of oxygen that gets to damaged tissue and speed up healing. Regular air is about 21 percent oxygen. But inside the HBOT chamber the atmospheric pressure can be increased to up to three times normal pressure at sea level and the patient breathes pure oxygen through a mask; blood becomes saturated with much higher levels of oxygen. This can defuse through the damaged capillaries of a wound and promote healing.
The trial
Efrati’s clinical trials started in December 2020, barely a year after SARS-CoV-2 had first appeared in Israel. Patients who’d experienced cognitive issues after having COVID received 40 sessions in the chamber over a period of 60 days. In each session, they spent 90 minutes breathing through a mask at two atmospheres of pressure. While inside, they performed mental exercises to train the brain. The only difference between the two groups of patients was that one breathed pure oxygen while the other group breathed normal air. No one knew who was receiving which level of oxygen.
The results were striking. Before and after fMRIs showed significant repair of damaged tissue in the brain and functional cognition tests improved substantially among those who received pure oxygen. Importantly, 80 percent of patients said they felt back to “normal,” but Efrati says they didn't include patient evaluation in the paper because there was no baseline data to show how they functioned before COVID. After the study was completed, the placebo group was offered a new round of treatments using 100 percent oxygen, and the team saw similar results.
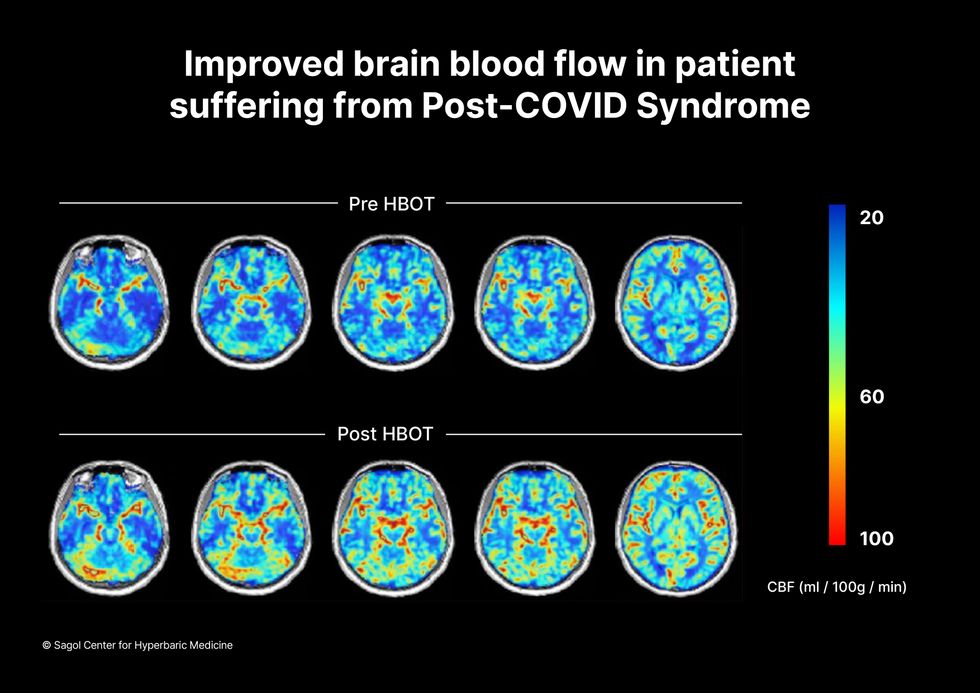
Scans show improved blood flow in a patient suffering from Long Covid.
Sagol Center for Hyperbaric Medicine
Efrati's use of HBOT is part of an emerging geroscience approach to diseases associated with aging. These researchers see systems dysfunctions that are common to several diseases, such as inflammation, which has been shown to play a role in micro infarcts, heart disease and Alzheimer’s disease. Preliminary research suggests that HBOT can retard some underlying mechanisms of aging, which might address several medical conditions. However, the drug approval process is set up to regulate individual disease, not conditions as broad as aging, and so they concentrate on treating the low hanging fruit: disorders where effective treatments currently are limited and success might be demonstrated.
The key to HBOT's effectiveness is something called the hyperoxic-hypoxic paradox where a body does not react to an increase in available oxygen, only to a decrease, regardless of the starting point. That danger signal has a powerful effect on gene expression, resulting in changes in metabolism, and the proliferation of stem cells. That occurs with each cycle of 20 minutes of pure oxygen followed by 5 minutes of regular air circulating through the masks, while the chamber remains pressurized. The high levels of oxygen in the blood provide the fuel necessary for tissue regeneration.
The hyperbaric chamber that Efrati has built can hold a dozen patients and attending medical staff. Think of it as a pressurized airplane cabin, only with much more space than even in first class. In the U.S., people think of HBOT as “a sack of air or some tube that you can buy on Amazon” or find at a health spa. “That is total bullshit,” Efrati says. “It has to be a medical class center where a physician can lose their license if they are not operating it properly.”

Shai Efrati
Alexander Charney, a research psychiatrist at the Icahn School of Medicine at Mount Sinai in New York City, calls Efrati’s study thoughtful and well designed. But it demands a lot from patients with its intense number of sessions. Those types of regimens have proven difficult to roll out to large numbers of patients. Still, the results are intriguing enough to merit additional trials.
John J. Miller, a physician and editor in chief of Psychiatric Times, has seen “many physicians that use hyperbaric oxygen for various brain disorders such as TBI.” He is intrigued by Efrati's work and believes the approach “has great potential to help patients with long COVID whose symptoms are related to brain tissue changes.”
Efrati believes so much in the power of the hyperoxic-hypoxic paradox to heal a variety of tissue injuries that he is leading the medical advisory board at Aviv Clinic, an international network of clinics that are delivering HBOT treatments based on research conducted in Israel. His goal is to silence doubters by quickly opening about 50 such clinics worldwide, based on the model of standalone dialysis clinics in the United States. Sagol Center is treating 300 patients per day, and clinics have opened in Florida and Dubai. There are plans to open another in Manhattan.
Catching colds may help protect kids from Covid
A new study shows that the immune system's response to colds can help prepare it to defend against COVID-19 - but only in the very young.
A common cold virus causes the immune system to produce T cells that also provide protection against SARS-CoV-2, according to new research. The study, published last month in PNAS, shows that this effect is most pronounced in young children. The finding may help explain why most young people who have been exposed to the cold-causing coronavirus have not developed serious cases of COVID-19.
One curiosity stood out in the early days of the COVID-19 pandemic – why were so few kids getting sick. Generally young children and the elderly are the most vulnerable to disease outbreaks, particularly viral infections, either because their immune systems are not fully developed or they are starting to fail.
But solid information on the new infection was so scarce that many public health officials acted on the precautionary principle, assumed a worst-case scenario, and applied the broadest, most restrictive policies to all people to try to contain the coronavirus SARS-CoV-2.
One early thought was that lockdowns worked and kids (ages 6 months to 17 years) simply were not being exposed to the virus. So it was a shock when data started to come in showing that well over half of them carried antibodies to the virus, indicating exposure without getting sick. That trend grew over time and the latest tracking data from the CDC shows that 96.3 percent of kids in the U.S. now carry those antibodies.
Antibodies are relatively quick and easy to measure, but some scientists are exploring whether the reactions of T cells could serve as a more useful measure of immune protection.
But that couldn't be the whole story because antibody protection fades, sometimes as early as a month after exposure and usually within a year. Additionally, SARS-CoV-2 has been spewing out waves of different variants that were more resistant to antibodies generated by their predecessors. The resistance was so significant that over time the FDA withdrew its emergency use authorization for a handful of monoclonal antibodies with earlier approval to treat the infection because they no longer worked.
Antibodies got most of the attention early on because they are part of the first line response of the immune system. Antibodies can bind to viruses and neutralize them, preventing infection. They are relatively quick and easy to measure and even manufacture, but as SARS-CoV-2 showed us, often viruses can quickly evolve to become more resistant to them. Some scientists are exploring whether the reactions of T cells could serve as a more useful measure of immune protection.
Kids, colds and T cells
T cells are part of the immune system that deals with cells once they have become infected. But working with T cells is much more difficult, takes longer, and is more expensive than working with antibodies. So studies often lags behind on this part of the immune system.
A group of researchers led by Annika Karlsson at the Karolinska Institute in Sweden focuses on T cells targeting virus-infected cells and, unsurprisingly, saw that they can play a role in SARS-CoV-2 infection. Other labs have shown that vaccination and natural exposure to the virus generates different patterns of T cell responses.
The Swedes also looked at another member of the coronavirus family, OC43, which circulates widely and is one of several causes of the common cold. The molecular structure of OC43 is similar to its more deadly cousin SARS-CoV-2. Sometimes a T cell response to one virus can produce a cross-reactive response to a similar protein structure in another virus, meaning that T cells will identify and respond to the two viruses in much the same way. Karlsson looked to see if T cells for OC43 from a wide age range of patients were cross-reactive to SARS-CoV-2.
And that is what they found, as reported in the PNAS study last month; there was cross-reactive activity, but it depended on a person’s age. A subset of a certain type of T cells, called mCD4+,, that recognized various protein parts of the cold-causing virus, OC43, expressed on the surface of an infected cell – also recognized those same protein parts from SARS-CoV-2. The T cell response was lower than that generated by natural exposure to SARS-CoV-2, but it was functional and thus could help limit the severity of COVID-19.
“One of the most politicized aspects of our pandemic response was not accepting that children are so much less at risk for severe disease with COVID-19,” because usually young children are among the most vulnerable to pathogens, says Monica Gandhi, professor of medicine at the University of California San Francisco.
“The cross-reactivity peaked at age six when more than half the people tested have a cross-reactive immune response,” says Karlsson, though their sample is too small to say if this finding applies more broadly across the population. The vast majority of children as young as two years had OC43-specific mCD4+ T cell responses. In adulthood, the functionality of both the OC43-specific and the cross-reactive T cells wane significantly, especially with advanced age.
“Considering that the mortality rate in children is the lowest from ages five to nine, and higher in younger children, our results imply that cross-reactive mCD4+ T cells may have a role in the control of SARS-CoV-2 infection in children,” the authors wrote in their paper.
“One of the most politicized aspects of our pandemic response was not accepting that children are so much less at risk for severe disease with COVID-19,” because usually young children are among the most vulnerable to pathogens, says Monica Gandhi, professor of medicine at the University of California San Francisco and author of the book, Endemic: A Post-Pandemic Playbook, to be released by the Mayo Clinic Press this summer. The immune response of kids to SARS-CoV-2 stood our expectations on their head. “We just haven't seen this before, so knowing the mechanism of protection is really important.”
Why the T cell immune response can fade with age is largely unknown. With some viruses such as measles, a single vaccination or infection generates life-long protection. But respiratory tract infections, like SARS-CoV-2, cause a localized infection - specific to certain organs - and that response tends to be shorter lived than systemic infections that affect the entire body. Karlsson suspects the elderly might be exposed to these localized types of viruses less often. Also, frequent continued exposure to a virus that results in reactivation of the memory T cell pool might eventually result in “a kind of immunosenescence or immune exhaustion that is associated with aging,” Karlsson says. https://leaps.org/scientists-just-started-testing-a-new-class-of-drugs-to-slow-and-even-reverse-aging/particle-3 This fading protection is why older people need to be repeatedly vaccinated against SARS-CoV-2.
Policy implications
Following the numbers on COVID-19 infections and severity over the last three years have shown us that healthy young people without risk factors are not likely to develop serious disease. This latest study points to a mechanism that helps explain why. But the inertia of existing policies remains. How should we adjust policy recommendations based on what we know today?
The World Health Organization (WHO) updated their COVID-19 vaccination guidance on March 28. It calls for a focus on vaccinating and boosting those at risk for developing serious disease. The guidance basically shrugged its shoulders when it came to healthy children and young adults receiving vaccinations and boosters against COVID-19. It said the priority should be to administer the “traditional essential vaccines for children,” such as those that protect against measles, rubella, and mumps.
“As an immunologist and a mother, I think that catching a cold or two when you are a kid and otherwise healthy is not that bad for you. Children have a much lower risk of becoming severely ill with SARS-CoV-2,” says Karlsson. She has followed public health guidance in Sweden, which means that her young children have not been vaccinated, but being older, she has received the vaccine and boosters. Gandhi and her children have been vaccinated, but they do not plan on additional boosters.
The WHO got it right in “concentrating on what matters,” which is getting traditional childhood immunizations back on track after their dramatic decline over the last three years, says Gandhi. Nor is there a need for masking in schools, according to a study from the Catalonia region of Spain. It found “no difference in masking and spread in schools,” particularly since tracking data indicate that nearly all young people have been exposed to SARS-CoV-2.
Both researchers lament that public discussion has overemphasized the quickly fading antibody part of the immune response to SARS-CoV-2 compared with the more durable T cell component. They say developing an efficient measure of T cell response for doctors to use in the clinic would help to monitor immunity in people at risk for severe cases of COVID-19 compared with the current method of toting up potential risk factors.
In this week's Friday Five: The eyes are the windows to the soul - and biological aging?
Plus, what bean genes mean for health and the planet, a breathing practice that could lower levels of tau proteins in the brain, AI beats humans at assessing heart health, and the benefits of "nature prescriptions"
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on new scientific theories and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the stories covered this week:
- The eyes are the windows to the soul - and biological aging?
- What bean genes mean for health and the planet
- This breathing practice could lower levels of tau proteins
- AI beats humans at assessing heart health
- Should you get a nature prescription?