For patients with macular degeneration, new hope for restored sight

An implant, combined with the glasses and tiny video camera modeled in this photo, could improve the eyesight of millions of people with degenerative eye diseases in the coming years.
For millions of people with macular degeneration, treatment options are slim. The disease causes loss of central vision, which allows us to see straight ahead, and is highly dependent on age, with people over 75 at approximately 30% risk of developing the disorder. The BrightFocus Foundation estimates 11 million people in the U.S. currently have one of three forms of the disease.
Recently, ophthalmologists including Daniel Palanker at Stanford University published research showing advances in the PRIMA retinal implant, which could help people with advanced, age-related macular degeneration regain some of their sight. In a feasibility study, five patients had a pixelated chip implanted behind the retina, and three were able to see using their remaining peripheral vision and—thanks to the implant—their partially restored central vision at the same time.
Should people with macular degeneration be excited about these results?
“Every week, if not every day, patients come to me with this question because it's devastating when they lose their central vision,” says retinal surgeon Lynn Huang. About 40% of her patients have macular degeneration. Huang tells them that these implants, along with new medications and stem cell therapies, could be useful in the coming years.
“The goal here is to replace the missing photoreceptors with photovoltaic pixels, basically like little solar panels,” Palanker says.
That implant, a pixelated chip, works together with a tiny video camera on a specially designed pair of eyeglasses, which can be adjusted for each patient’s prescription. The video camera relays processed images to the chip, which electrically stimulates inner retinal neurons. These neurons, in turn, relay information to the brain’s visual cortex through the optic nerve. The chip restores patients’ central sight, but not completely. The artificial vision is basically monochromatic (whitish-yellowish) and fairly blurry; patients were still legally blind even after the implant, except when using a zoom function on the camera, but those with proper chip placement could make out large letters.
“The goal here is to replace the missing photoreceptors with photovoltaic pixels, basically like little solar panels,” Palanker says. These pixels, located on the implanted chip, convert light into pulsed electrical currents that stimulate retinal neurons. In time, Palanker hopes to improve the chips, resulting in bigger boosts to visual acuity.
The pixelated chips are surgically implanted during a process Palanker admits is still “a surgical learning curve.” In the study, three chips were implanted correctly, one was placed incorrectly, and another patient’s chip moved after the procedure; he did not follow post-surgical recommendations. One patient passed away during the study for unrelated reasons.
University of Maryland retinal specialist Kenneth Taubenslag, who was not involved in the study, said that subretinal surgeries have become less common in recent years, but expects implants to spur improvements in these techniques. “I think as people get more experience, [they’ll] probably get more reliable placement of the implant,” he said, pointing out that even the patient with the misplaced chip was able to gain some light perception, if not the same visual acuity as other patients.
Retinal implants have come under scrutiny lately. IEEE Spectrum reported that Second Sight, manufacturer of the Argus II implant used for people with retinitis pigmentosa, a genetic disease that causes vision loss, would no longer support the product. After selling hundreds of the implants at $150,000 apiece, company leaders announced they’d “decided to pursue an orderly wind down” of Second Sight in March 2020 in the wake of financial issues. Last month, the company announced a merger, shifting its focus to a new retinal implant, raising questions for patients who have Argus II implants.
Retinal surgeon Eugene de Juan of the University of California, San Francisco, was involved with early studies of the Argus implants, though his participation ended over a decade ago, before the device was marketed by Second Sight. He says he would consider recommending future implants to patients with macular degeneration, given the promise of the technology and the lack of other alternatives.
“I tell my patients that this is an area of active research and development, and it's getting better and better, so let's not give up hope,” de Juan says. He believes cautious optimism for Palanker’s implant is appropriate: “It's not the first, it's not the only, but it's a good approach with a good team.”
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
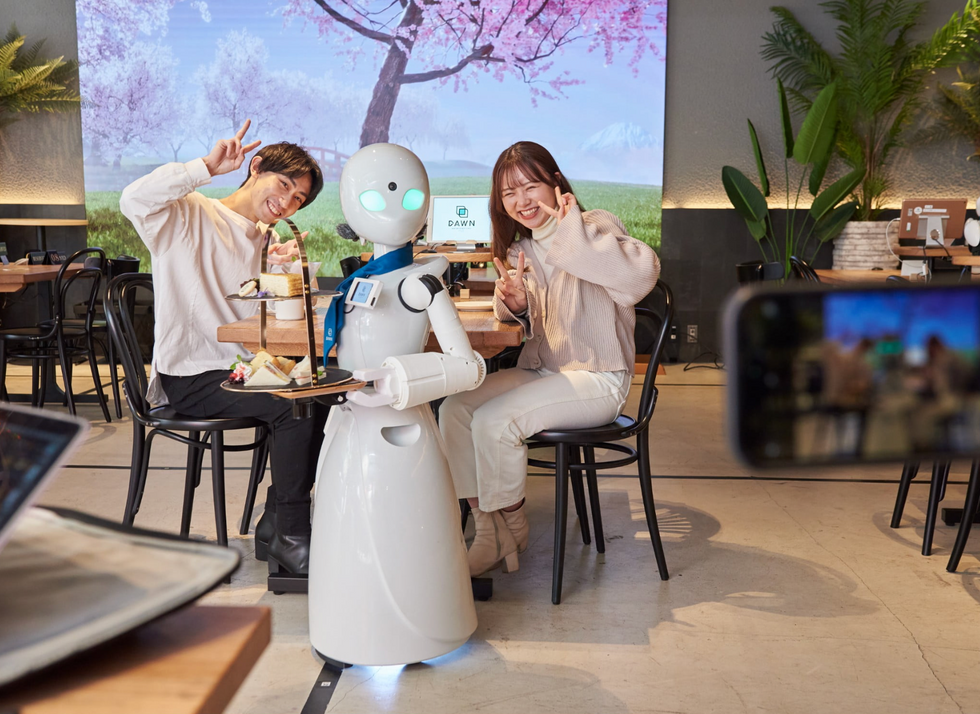
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

