Sexually Transmitted Infections are on the rise. This drug could stop them.

Cases of gonorrhea, chlamydia and syphilis soared last year, but researchers are finding that a drug known as doxy seems to reduce the number of infections.
Sexually transmitted infections (STIs) are surging across the U.S. to 2.5 million cases in 2021 according to preliminary data from the CDC. A new prevention and treatment strategy now in clinical trials may provide a way to get a handle on them.
It's easy to overlook the soaring rates of gonorrhea, chlamydia, and syphilis because most of those infections have few or no symptoms and can be identified only through testing. But left untreated, they can lead to serious damage to nerves and tissue, resulting in infertility, blindness, and dementia. Infants developing in utero are particularly vulnerable.
Covid-19 played havoc with regular medical treatment and preventive care for many health problems, including STIs. After formal lockdowns ended, many people gradually became more socially engaged, with increases in sexual activity, and may have prioritized these activities over getting back in touch with their doctors.
A second blow to controlling STIs is that family planning clinics are closing left and right because of the Dobbs decision and legislation in many states that curtailed access to an abortion. Discussion has focused on abortion, but those same clinics also play a vital role in the diagnosis and treatment of STIs.
Routine public health is the neglected stepchild of medicine. It is called upon in times of crisis but as that crisis resolves, funding dries up. Labs have atrophied and personnel have been redirected to Covid, “so access to routine screening for STIs has been decimated,” says Jennifer Mahn, director of sexual and clinical health with the National Coalition of STD Directors.
A preview of what we likely are facing comes from Iowa. In 2017, the state legislature restricted funding to family health clinics in four counties, which closed their doors. A year later the statewide rate of gonorrhea skyrocketed from 83 to 153.7 cases per 100,000 people. “Iowa counties with clinic closures had a significantly larger increase,” according to a study published in JAMA. That scenario likely is playing out in countless other regions where access to sexual health care is shrinking; it will be many months before we have the data to know for sure.
A decades-old antibiotic finds a new purpose
Using drugs to protect against HIV, either as post exposure prophylaxis (PEP) or pre-exposure prophylaxis (PrEP), has proven to be quite successful. Researchers wondered if the same approach might be applied to other STIs. They focused on doxycycline, or doxy for short. One of the most commonly prescribed antibiotics in the U.S., it’s a member of the tetracycline family that has been on the market since 1967. It is so safe that it’s used to treat acne.
Two small studies using doxy suggested that it could work to prevent STIs. A handful of clinical trials by different researchers and funding sources set out to generate the additional evidence needed to prove their hypothesis and change the standard of care.
Senior researcher Victor Omollo, with the Kenya Medical Research Institute, noted, “These are prevention interventions that women can control on their own without having to seek or get consent from another person,” as is the case with condom use.
The first with results is the DoxyPEP study, conducted at two sexual health clinics in San Francisco and Seattle. It drew from a mix of transgender women and men who have sex with men, who had at least one diagnosed STI over the last year. The researchers divided the participants into two groups: one with people who were already HIV-positive and engaged in care, while the other group consisted of people who were on PrEP to prevent infection with HIV. For the active part of the study, a subset of the participants received doxy, and the rest of the participants did not.
The researchers intentionally chose to do the study in a population at the highest risk of having STIs, who were very health oriented, and “who were getting screened every three months or so as part of their PrEP program or their HIV care program,” says Connie Celum, a senior researcher at the University of Washington on the study.
Each member of the active group was given a supply of doxy and asked to take two pills within 72 hours of having sex where a condom was not used. The study was supposed to run for two years but, in May, it stopped halfway through, when a safety monitoring board looked at the data and recommended that it would be unethical to continue depriving the control group of the drug’s benefits.
Celum presented these preliminary results from the DoxyPEP study in July at the International AIDS Conference in Montreal. “We saw about a 56 percent reduction in gonorrhea, about 80 percent reduction in chlamydia and syphilis, so very significant reductions, and this is on a per quarter basis,” she told a later webinar.
In Kenya, another study is following a group of cisgender women who are taking the same two-pill regimen to prevent HIV, and the data from this research should become available in 2023. Senior researcher Victor Omollo, with the Kenya Medical Research Institute, noted that “these are prevention interventions that women can control on their own without having to seek or get consent from another person,” as is the case with condom use, another effective prevention tool.
Antibiotic resistance
Antibiotic resistance is a potentially big concern. About 25 percent of gonorrhea strains circulating in the U.S. are resistant to the tetracycline class of drugs, including doxy; rates are higher elsewhere. But resistance often is a matter of degree and can be overcome with a larger or longer dose of the drug, or perhaps with a switch to another drug or a two-drug combination.
Research has shown that an established bacterial infection is more difficult to treat because it is part of a biofilm, which can leave only a small portion or perhaps none of the cell surface exposed to a drug. But a new infection, even one where the bacteria is resistant to a drug, might still be vulnerable to that drug if it's used before the bacterial biofilm can be established. Preliminary data suggests that may be the case with doxyPEP and drug resistant gonorrhea; some but not all new drug resistant infections might be thwarted if they’re treated early enough.
“There are some tradeoffs” to these interventions, Celum says, and people may disagree on the cost of increased resistance balanced against the benefits of treating the STIs and reducing their spread within the community.
Resistance does not seem to be an issue yet for chlamydia and syphilis even though doxy has been a recommended treatment for decades, but a remaining question is whether broader use of doxy will directly worsen antibiotic resistance in gonorrhea, or promote it in other STIs. And how will it affect the gut microbiome?
In addition, Celum notes that we need to understand whether doxy will generate mutations in other bacteria that might contribute to drug resistance for gonorrhea, chlamydia or syphilis. The studies underway aim to provide data to answer these questions.
“There are some tradeoffs” to these interventions, Celum says, and people may disagree on the cost of increased resistance balanced against the benefits of treating the STIs and reducing their spread within the community. That might affect doctors' willingness to prescribe the drug.
Turning research into action
The CDC makes policy recommendations for prevention services such as taking doxy, requiring some and leaving others optional. Celum says the CDC will be reviewing information from her trial at a meeting in December, but probably will wait until that study is published before making recommendations, likely in 2023. The San Francisco Department of Public Health issued its own guidance on October 20th and anecdotally, some doctors around the country are beginning to issue prescriptions for doxy to select patients.
About half of new STIs occur in young people ages 15 to 24, a group that is least likely to regularly see a doctor. And sexual health remains a great taboo for many people who don't want such information on their health record for prying parents, employers or neighbors to find out.
“People will go out of their way and travel extensive distances just to avoid that,” says Mahn, the National Coalition director. “People identify locations where they feel safe, where they feel welcome, where they don't feel judged,” Mahn explains, such as community and family planning clinics. They understand those issues and have fees that vary depending on a person’s ability to pay.
Given that these clinics already are understaffed and underfunded, they will be hard pressed to expand services covering the labor intensive testing and monitoring of a doxyPEP regimen. Sexual health clinics don't even have a separate line item in the federal budget for health. That is something the National Association of STI Directors is pushing for in D.C.
DoxyPEP isn't a panacea, and it isn't for everyone. “We really want to try to reach that population who is most likely going to have an STI in the next year,” says Celum, “Because that's where you are going to have the biggest impact.”
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
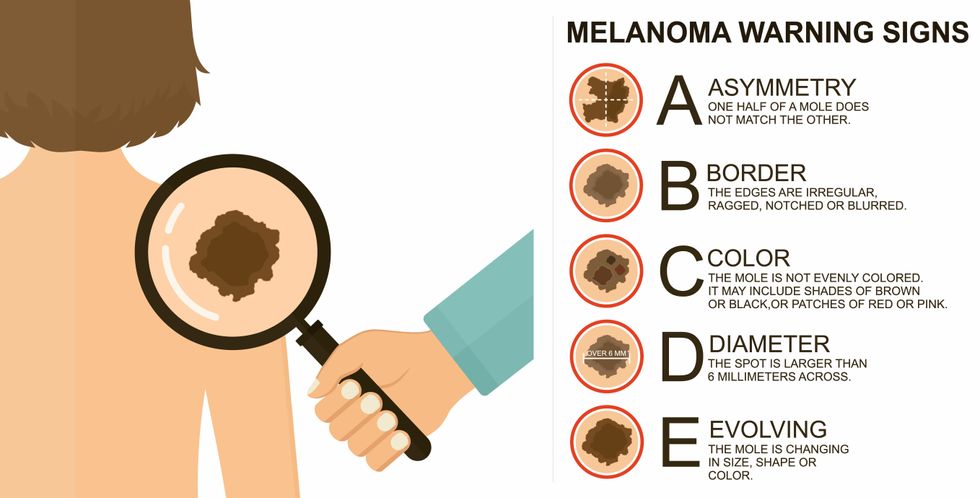
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

