Is China Winning the Innovation Race?

Flags of America and China illuminated by light bulbs with a question mark between them.
Over the past two millennia, Chinese ingenuity has spawned some of humanity's most consequential inventions. Without gunpowder, guns, bombs, and rockets; without paper, printing, and money printed on paper; and without the compass, which enabled ships to navigate the open ocean, modern civilization might never have been born.
Today, a specter is haunting the developed world: Chinese innovation dominance. And the results have been so spectacular that the United States feels its preeminence threatened.
Yet China lapsed into cultural and technological stagnation during the Qing dynasty, just as the Scientific Revolution was transforming Europe. Western colonial incursions and a series of failed rebellions further sapped the Celestial Empire's capacity for innovation. By the mid-20th century, when the Communist triumph led to a devastating famine and years of bloody political turmoil, practically the only intellectual property China could offer for export was Mao's Little Red Book.
After Deng Xiaoping took power in 1978, launching a transition from a rigidly planned economy to a semi-capitalist one, China's factories began pumping out goods for foreign consumption. Still, originality remained a low priority. The phrase "Made in China" came to be synonymous with "cheap knockoff."
Today, however, a specter is haunting the developed world: Chinese innovation dominance. It first wafted into view in 2006, when the government announced an "indigenous innovation" campaign, dedicated to establishing China as a technology powerhouse by 2020—and a global leader by 2050—as part of its Medium- and Long-Term National Plan for Science and Technology Development. Since then, an array of initiatives have sought to unleash what pundits often call the Chinese "tech dragon," whether in individual industries, such as semiconductors or artificial intelligence, or across the board (as with the Made in China 2025 project, inaugurated in 2015). These efforts draw on a well-stocked bureaucratic arsenal: state-directed financing; strategic mergers and acquisitions; competition policies designed to boost domestic companies and hobble foreign rivals; buy-Chinese procurement policies; cash incentives for companies to file patents; subsidies for academic researchers in favored fields.
The results have been spectacular—so much so that the United States feels its preeminence threatened. Voices across the political spectrum are calling for emergency measures, including a clampdown on technology transfers, capital investment, and Chinese students' ability to study abroad. But are the fears driving such proposals justified?
"We've flipped from thinking China is incapable of anything but imitation to thinking China is about to eat our lunch," says Kaiser Kuo, host of the Sinica podcast at supchina.com, who recently returned to the U.S after 20 years in Beijing—the last six as director of international communications for the tech giant Baidu. Like some other veteran China-watchers, Kuo believes neither extreme reflects reality. "We're in as much danger now of overestimating China's innovative capacity," he warns, "as we were a few years ago of underestimating it."
A Lab and Tech-Business Bonanza
By many measures, China's innovation renaissance is mind-boggling. Spending on research and development as a percentage of gross domestic product nearly quadrupled between 1996 and 2016, from .56 percent to 2.1 percent; during the same period, spending in the United States rose by just .3 percentage points, from 2.44 to 2.79 percent of GDP. China is now second only to the U.S. in total R&D spending, accounting for 21 percent of the global total of $2 trillion, according to a report released in January by the National Science Foundation. In 2016, the number of scientific publications from China exceeded those from the U.S. for the first time, by 426,000 to 409,000. Chinese researchers are blazing new trails on the frontiers of cloning, stem cell medicine, gene editing, and quantum computing. Chinese patent applications have soared from 170,000 to nearly 3 million since 2000; the country now files almost as many international patents as the U.S. and Japan, and more than Germany and South Korea. Between 2008 and 2017, two Chinese tech firms—Huawei and ZTE—traded places as the world's top patent filer in six out of nine years.
"China is still in its Star Trek phase, while we're in our Black Mirror phase." Yet there are formidable barriers to China beating America in the innovation race—or even catching up anytime soon.
Accompanying this lab-based ferment is a tech-business bonanza. China's three biggest internet companies, Baidu, Alibaba Group and Tencent Holdings (known collectively as BAT), have become global titans of search, e-commerce, mobile payments, gaming, and social media. Da-Jiang Innovations in Science and Technology (DJI) controls more than 70 percent of the world's commercial drone market. Of the planet's 262 "unicorns" (startups worth more than a billion dollars), about one-third are Chinese. The country attracted $77 billion in venture capital investment between 2014 and 2016, according to Fortune, and is now among the top three markets for VC in emerging technologies including AI, virtual reality, autonomous vehicles, and 3D printing.
These developments have fueled a buoyant techno-optimism in China that contrasts sharply with the darker view increasingly prevalent in the West—in part, perhaps, because China's historic limits on civil liberties have inured the populace to the intrusive implications of, say, facial recognition technology or social-credit software, which are already being used to tighten government control. "China is still in its Star Trek phase, while we're in our Black Mirror phase," Kuo observes. By contrast with Americans' ambivalent attitudes toward Facebook founder Mark Zuckerberg or Amazon's Jeff Bezos, he adds, most Chinese regard tech entrepreneurs like Baidu's Robin Li and Alibaba's Jack Ma as "flat-out heroes."
Yet there are formidable barriers to China beating America in the innovation race—or even catching up anytime soon. Many are catalogued in The Fat Tech Dragon, a 2017 monograph by Scott Kennedy, deputy director of the Freeman Chair in China Studies and director of the Project on Chinese Business and Political Economy at the Center for Strategic and International Studies. Among the obstacles, Kennedy writes, are "an education system that encourages deference to authority and does not prepare students to be creative and take risks, a financial system that disproportionately funnels funds to undeserving state-owned enterprises… and a market structure where profits can be made through a low-margin, high-volume strategy or through political connections."
China's R&D money, Kennedy points out, is mostly showered on the "D": of the $209 billion spent in 2015, only 5 percent went toward basic research, 10.8 percent toward applied research, and a massive 84.2 percent toward development. While fully half of venture capital in the States goes to early-stage startups, the figure for China is under 20 percent; true "angel" investors are scarce. Likewise, only 21 percent of Chinese patents are for original inventions, as opposed to tweaks of existing technologies. Most problematic, the domestic value of patents in China is strikingly low. In 2015, the country's patent licensing generated revenues of just $1.75 billion, compared to $115 billion for IP licensing in the U.S. in 2012 (the most recent year for which data is available). In short, Kennedy concludes, "China may now be a 'large' IP country, but it is still a 'weak' one."
"[The Chinese] are trying very hard to keep the economy from crashing, but it'll happen eventually. Then there will be a major, major contraction."
Anne Stevenson-Yang, co-founder and research director of J Capital Research, and a leading China analyst, sees another potential stumbling block: the government's obsession with neck-snapping GDP growth. "What China does is to determine, 'Our GDP growth will be X,' and then it generates enough investment to create X," Stevenson-Yang explains. To meet those quotas, officials pour money into gigantic construction projects, creating the empty "ghost cities" that litter the countryside, or subsidize industrial production far beyond realistic demand. "It's the ultimate Ponzi-scheme economy," she says, citing as examples the Chinese cellphone and solar industries, which ballooned on state funding, flooded global markets with dirt-cheap products, thrived just long enough to kill off most of their overseas competitors, and then largely collapsed. Such ventures, Stevenson-Yang notes, have driven China's debt load perilously high. "They're trying very hard to keep the economy from crashing, but it'll happen eventually," she predicts. "Then there will be a major, major contraction."
"An Intensifying Race Toward Techno-Nationalism"
The greatest vulnerability of the Chinese innovation boom may be that it still depends heavily on imported IP. "Over the last few years, China has placed its bets on a combination of global knowledge sourcing and indigenous technology development," says Dieter Ernst, a senior fellow at the Centre for International Governance Innovation in Waterloo, Canada, and the East-West Center in Honolulu, who has served as an Asia advisor for the U.N. and the World Bank. Aside from international journals (and, occasionally, industrial espionage), Chinese labs and corporations obtain non-indigenous knowledge in a number of ways: by paying licensing fees; recruiting Chinese scientists and engineers who've studied or worked abroad; hiring professionals from other countries; or acquiring foreign companies. And though enforcement of IP laws has improved markedly in recent years, foreign businesses are often pressured to provide technology transfers in exchange for access to markets.
Many of China's top tech entrepreneurs—including Ma, Li, and Alibaba's Joseph Tsai—are alumni of U.S. universities, and, as Kuo puts it, "big fans of all things American." Unfortunately, however, Americans are ever less likely to be fans of China, thanks largely to that country's sometimes predatory trade practices—and also to what Ernst calls "an intensifying race toward techno-nationalism." With varying degrees of bellicosity and consistency, leaders of both U.S. parties embrace elements of the trend, as do politicians (and voters) across much of Europe. "There's a growing consensus that China is poised to overtake us," says Ernst, "and that we need to design policies to obstruct its rise."
One of the foremost liberal analysts supporting this view is Lee Branstetter, a professor of economics and public policy at Carnegie Mellon University and former senior economist on President Barack Obama's Council of Economic Advisors. "Over the decades, in a systematic and premeditated fashion, the Chinese government and its state-owned enterprises have worked to extract valuable technology from foreign multinationals, with an explicit goal of eventually displacing those leading multinationals with successful Chinese firms in global markets," Branstetter wrote in a 2017 report to the United States Trade Representative. To combat such "forced transfers," he suggested, laws could be passed empowering foreign governments to investigate coercive requests and block any deemed inappropriate—not just those involving military-related or crucial infrastructure technology, which current statutes cover. Branstetter also called for "sharply" curtailing Chinese students' access to Western graduate programs, as a way to "get policymakers' attention in Beijing" and induce them to play fair.
Similar sentiments are taking hold in Congress, where the Foreign Investment Risk Review Modernization Act—aimed at strengthening the process by which the Committee on Foreign Investment in the United States reviews Chinese acquisition of American technologies—is expected to pass with bipartisan support, though its harsher provisions were softened due to objections from Silicon Valley. The Trump Administration announced in May that it would soon take executive action to curb Chinese investments in U.S. tech firms and otherwise limit access to intellectual property. The State Department, meanwhile, imposed a one-year limit on visas for Chinese grad students in high-tech fields.
Ernst argues that such measures are motivated largely by exaggerated notions of China's ability to reach its ambitious goals, and by the political advantages that fearmongering confers. "If you look at AI, chip design and fabrication, robotics, pharmaceuticals, the gap with the U.S. is huge," he says. "Reducing it will take at least 10 or 15 years."
Cracking down on U.S. tech transfers to Chinese companies, Ernst cautions, will deprive U.S. firms of vital investment capital and spur China to retaliate, cutting off access to the nation's gargantuan markets; it will also push China to forge IP deals with more compliant nations, or revert to outright piracy. And restricting student visas, besides harming U.S. universities that depend on Chinese scholars' billions in tuition, will have a "chilling effect on America's ability to attract to researchers and engineers from all countries."
"It's not a zero-sum game. I don't think China is going to eat our lunch. We can sit down and enjoy lunch together."
America's own science and technology community, Ernst adds, considers it crucial to swap ideas with China's fast-growing pool of talent. The 2017 annual meeting of the Palo Alto-based Association for Advancement of Artificial Intelligence, he notes, featured a nearly equal number of papers by researchers in China and the U.S. Organizers postponed the meeting after discovering that the original date coincided with the Chinese New Year.
China's rising influence on the tech world carries upsides as well as downsides, Scott Kennedy observes. The country's successes in e-commerce, he says, "haven't damaged the global internet sector, but have actually been a spur to additional innovation and progress. By contrast, China's success in solar and wind has decimated the global sectors," due to state-mandated overcapacity. "When Chinese firms win through open competition, the outcome is constructive; when they win through industrial policy and protectionism, the outcome is destructive."
The solution, Kennedy and like-minded experts argue, is to discourage protectionism rather than engage in it, adjusting tech-transfer policy just enough to cope with evolving national-security concerns. Instead of trying to squelch China's innovation explosion, they say, the U.S. should seek ways to spread its potential benefits (as happened in previous eras with Japan and South Korea), and increase America's indigenous investments in tech-related research, education, and job training.
"It's not a zero-sum game," says Kaiser Kuo. "I don't think China is going to eat our lunch. We can sit down and enjoy lunch together."
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
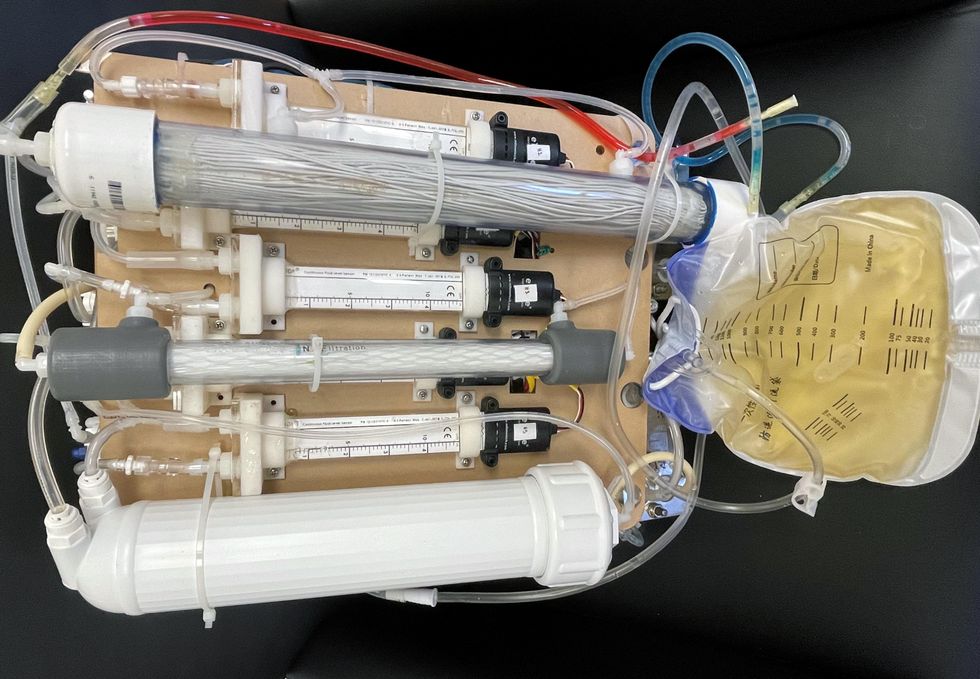
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
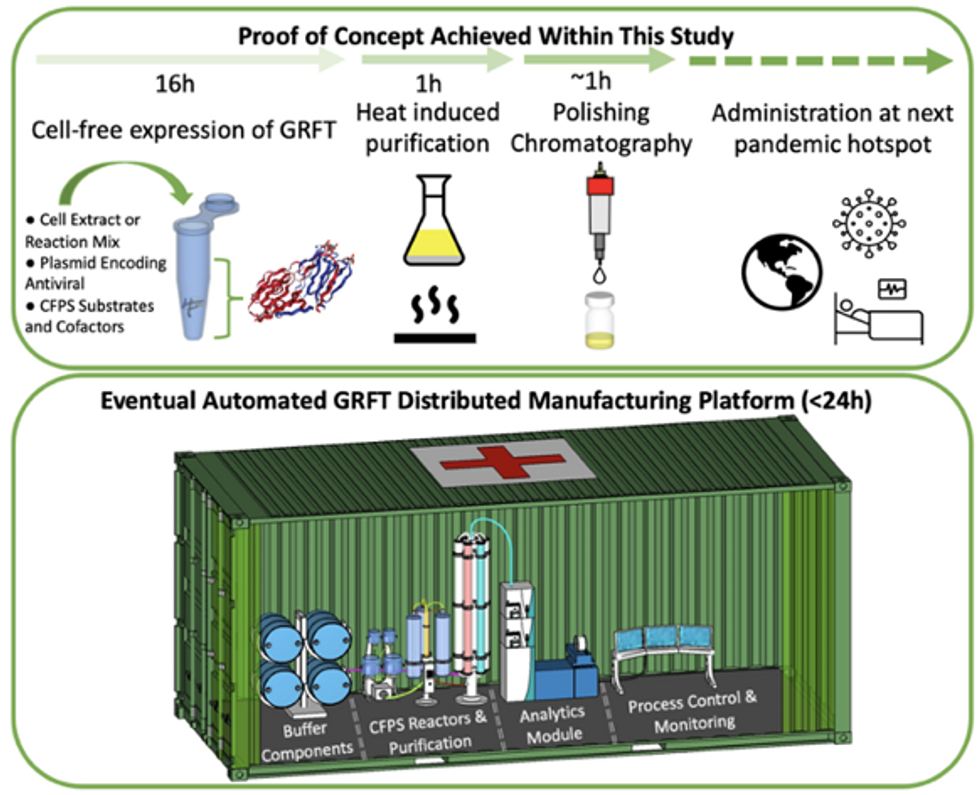
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.