Is Finding Out Your Baby’s Genetics A New Responsibility of Parenting?
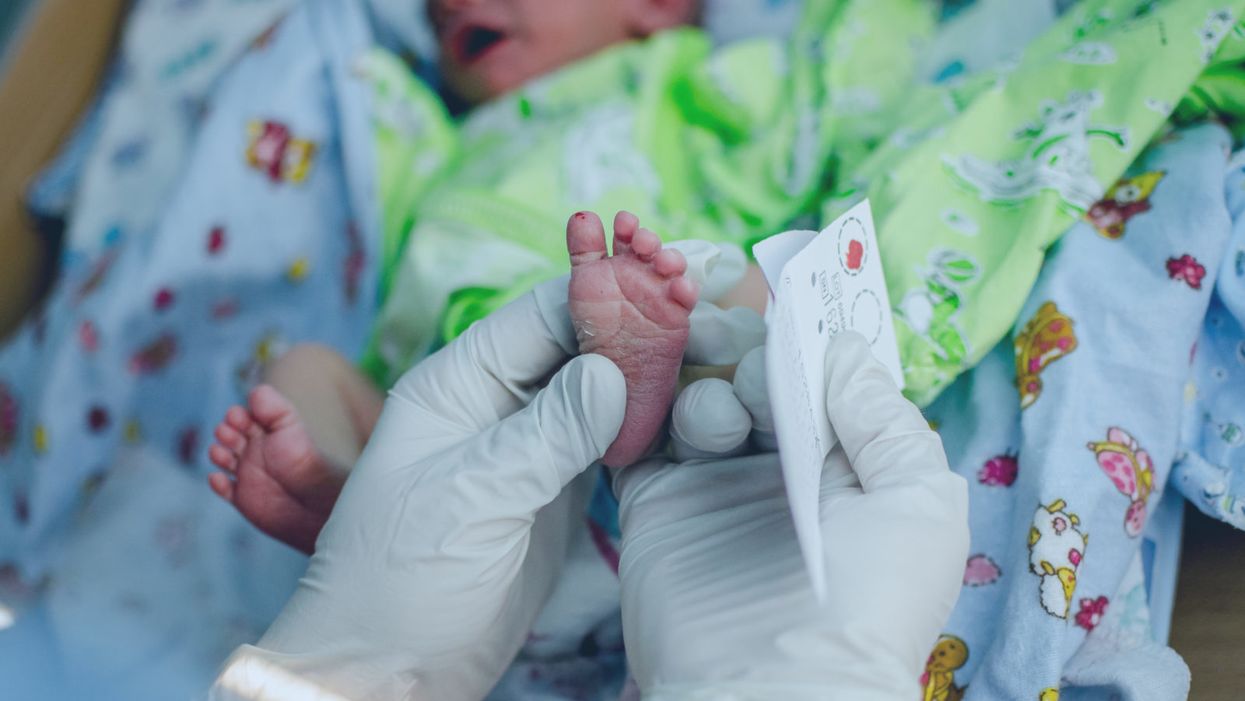
A doctor pricks the heel of a newborn for a blood test.
Hours after a baby is born, its heel is pricked with a lancet. Drops of the infant's blood are collected on a porous card, which is then mailed to a state laboratory. The dried blood spots are screened for around thirty conditions, including phenylketonuria (PKU), the metabolic disorder that kick-started this kind of newborn screening over 60 years ago. In the U.S., parents are not asked for permission to screen their child. Newborn screening programs are public health programs, and the assumption is that no good parent would refuse a screening test that could identify a serious yet treatable condition in their baby.
Learning as much as you can about your child's health might seem like a natural obligation of parenting. But it's an assumption that I think needs to be much more closely examined.
Today, with the introduction of genome sequencing into clinical medicine, some are asking whether newborn screening goes far enough. As the cost of sequencing falls, should parents take a more expansive look at their children's health, learning not just whether they have a rare but treatable childhood condition, but also whether they are at risk for untreatable conditions or for diseases that, if they occur at all, will strike only in adulthood? Should genome sequencing be a part of every newborn's care?
It's an idea that appeals to Anne Wojcicki, the founder and CEO of the direct-to-consumer genetic testing company 23andMe, who in a 2016 interview with The Guardian newspaper predicted that having newborns tested would soon be considered standard practice—"as critical as testing your cholesterol"—and a new responsibility of parenting. Wojcicki isn't the only one excited to see everyone's genes examined at birth. Francis Collins, director of the National Institutes of Health and perhaps the most prominent advocate of genomics in the United States, has written that he is "almost certain … that whole-genome sequencing will become part of new-born screening in the next few years." Whether that would happen through state-mandated screening programs, or as part of routine pediatric care—or perhaps as a direct-to-consumer service that parents purchase at birth or receive as a baby-shower gift—is not clear.
Learning as much as you can about your child's health might seem like a natural obligation of parenting. But it's an assumption that I think needs to be much more closely examined, both because the results that genome sequencing can return are more complex and more uncertain than one might expect, and because parents are not actually responsible for their child's lifelong health and well-being.
What is a parent supposed to do about such a risk except worry?
Existing newborn screening tests look for the presence of rare conditions that, if identified early in life, before the child shows any symptoms, can be effectively treated. Sequencing could identify many of these same kinds of conditions (and it might be a good tool if it could be targeted to those conditions alone), but it would also identify gene variants that confer an increased risk rather than a certainty of disease. Occasionally that increased risk will be significant. About 12 percent of women in the general population will develop breast cancer during their lives, while those who have a harmful BRCA1 or BRCA2 gene variant have around a 70 percent chance of developing the disease. But for many—perhaps most—conditions, the increased risk associated with a particular gene variant will be very small. Researchers have identified over 600 genes that appear to be associated with schizophrenia, for example, but any one of those confers only a tiny increase in risk for the disorder. What is a parent supposed to do about such a risk except worry?
Sequencing results are uncertain in other important ways as well. While we now have the ability to map the genome—to create a read-out of the pairs of genetic letters that make up a person's DNA—we are still learning what most of it means for a person's health and well-being. Researchers even have a name for gene variants they think might be associated with a disease or disorder, but for which they don't have enough evidence to be sure. They are called "variants of unknown (or uncertain) significance (VUS), and they pop up in most people's sequencing results. In cancer genetics, where much research has been done, about 1 in 5 gene variants are reclassified over time. Most are downgraded, which means that a good number of VUS are eventually designated benign.
While one parent might reasonably decide to learn about their child's risk for a condition about which nothing can be done medically, a different, yet still thoroughly reasonable, parent might prefer to remain ignorant so that they can enjoy the time before their child is afflicted.
Then there's the puzzle of what to do about results that show increased risk or even certainty for a condition that we have no idea how to prevent. Some genomics advocates argue that even if a result is not "medically actionable," it might have "personal utility" because it allows parents to plan for their child's future needs, to enroll them in research, or to connect with other families whose children carry the same genetic marker.
Finding a certain gene variant in one child might inform parents' decisions about whether to have another—and if they do, about whether to use reproductive technologies or prenatal testing to select against that variant in a future child. I have no doubt that for some parents these personal utility arguments are persuasive, but notice how far we've now strayed from the serious yet treatable conditions that motivated governments to set up newborn screening programs, and to mandate such testing for all.
Which brings me to the other problem with the call for sequencing newborn babies: the idea that even if it's not what the law requires, it's what good parents should do. That idea is very compelling when we're talking about sequencing results that show a serious threat to the child's health, especially when interventions are available to prevent or treat that condition. But as I have shown, many sequencing results are not of this type.
While one parent might reasonably decide to learn about their child's risk for a condition about which nothing can be done medically, a different, yet still thoroughly reasonable, parent might prefer to remain ignorant so that they can enjoy the time before their child is afflicted. This parent might decide that the worry—and the hypervigilence it could inspire in them—is not in their child's best interest, or indeed in their own. This parent might also think that it should be up to the child, when he or she is older, to decide whether to learn about his or her risk for adult-onset conditions, especially given that many adults at high familial risk for conditions like Alzheimer's or Huntington's disease choose never to be tested. This parent will value the child's future autonomy and right not to know more than they value the chance to prepare for a health risk that won't strike the child until 40 or 50 years in the future.
Parents are not obligated to learn about their children's risk for a condition that cannot be prevented, has a small risk of occurring, or that would appear only in adulthood.
Contemporary understandings of parenting are famously demanding. We are asked to do everything within our power to advance our children's health and well-being—to act always in our children's best interests. Against that backdrop, the need to sequence every newborn baby's genome might seem obvious. But we should be skeptical. Many sequencing results are complex and uncertain. Parents are not obligated to learn about their children's risk for a condition that cannot be prevented, has a small risk of occurring, or that would appear only in adulthood. To suggest otherwise is to stretch parental responsibilities beyond the realm of childhood and beyond factors that parents can control.
Should We Use Technologies to Enhance Morality?
Should we welcome biomedical technologies that could enhance our ability to tell right from wrong and improve behaviors that are considered immoral such as dishonesty, prejudice and antisocial aggression?
Our moral ‘hardware’ evolved over 100,000 years ago while humans were still scratching the savannah. The perils we encountered back then were radically different from those that confront us now. To survive and flourish in the face of complex future challenges our archaic operating systems might need an upgrade – in non-traditional ways.
Morality refers to standards of right and wrong when it comes to our beliefs, behaviors, and intentions. Broadly, moral enhancement is the use of biomedical technology to improve moral functioning. This could include augmenting empathy, altruism, or moral reasoning, or curbing antisocial traits like outgroup bias and aggression.
The claims related to moral enhancement are grand and polarizing: it’s been both tendered as a solution to humanity’s existential crises and bluntly dismissed as an armchair hypothesis. So, does the concept have any purchase? The answer leans heavily on our definition and expectations.
One issue is that the debate is often carved up in dichotomies – is moral enhancement feasible or unfeasible? Permissible or impermissible? Fact or fiction? On it goes. While these gesture at imperatives, trading in absolutes blurs the realities at hand. A sensible approach must resist extremes and recognize that moral disrupters are already here.
We know that existing interventions, whether they occur unknowingly or on purpose, have the power to modify moral dispositions in ways both good and bad. For instance, neurotoxins can promote antisocial behavior. The ‘lead-crime hypothesis’ links childhood lead-exposure to impulsivity, antisocial aggression, and various other problems. Mercury has been associated with cognitive deficits, which might impair moral reasoning and judgement. It’s well documented that alcohol makes people more prone to violence.
So, what about positive drivers? Here’s where it gets more tangled.
Medicine has long treated psychiatric disorders with drugs like sedatives and antipsychotics. However, there’s short mention of morality in the Diagnostic and Statistical Manual of Mental Disorders (DSM) despite the moral merits of pharmacotherapy – these effects are implicit and indirect. Such cases are regarded as treatments rather than enhancements.
It would be dangerously myopic to assume that moral augmentation is somehow beyond reach.
Conventionally, an enhancement must go beyond what is ‘normal,’ species-typical, or medically necessary – this is known as the ‘treatment-enhancement distinction.’ But boundaries of health and disease are fluid, so whether we call a procedure ‘moral enhancement’ or ‘medical treatment’ is liable to change with shifts in social values, expert opinions, and clinical practices.
Human enhancements are already used for a range of purported benefits: caffeine, smart drugs, and other supplements to boost cognitive performance; cosmetic procedures for aesthetic reasons; and steroids and stimulants for physical advantage. More boldly, cyborgs like Moon Ribas and Neil Harbisson are pushing transpecies boundaries with new kinds of sensory perception. It would be dangerously myopic to assume that moral augmentation is somehow beyond reach.
How might it work?
One possibility for shaping moral temperaments is with neurostimulation devices. These use electrodes to deliver a low-intensity current that alters the electromagnetic activity of specific neural regions. For instance, transcranial Direct Current Stimulation (tDCS) can target parts of the brain involved in self-awareness, moral judgement, and emotional decision-making. It’s been shown to increase empathy and valued-based learning, and decrease aggression and risk-taking behavior. Many countries already use tDCS to treat pain and depression, but evidence for enhancement effects on healthy subjects is mixed.
Another suggestion is targeting neuromodulators like serotonin and dopamine. Serotonin is linked to prosocial attributes like trust, fairness, and cooperation, but low activity is thought to motivate desires for revenge and harming others. It’s not as simple as indiscriminately boosting brain chemicals though. While serotonin is amenable to SSRIs, precise levels are difficult to measure and track, and there’s no scientific consensus on the “optimum” amount or on whether such a value even exists. Fluctuations due to lifestyle factors such as diet, stress, and exercise add further complexity. Currently, more research is needed on the significance of neuromodulators and their network dynamics across the moral landscape.
There are a range of other prospects. The ‘love drugs’ oxytocin and MDMA mediate pair bonding, cooperation, and social attachment, although some studies suggest that people with high levels of oxytocin are more aggressive toward outsiders. Lithium is a mood stabilizer that has been shown to reduce aggression in prison populations; beta-blockers like propranolol and the supplement omega-3 have similar effects. Increasingly, brain-computer interfaces augur a world of brave possibilities. Such appeals are not without limitations, but they indicate some ways that external tools can positively nudge our moral sentiments.
Who needs morally enhancing?
A common worry is that enhancement technologies could be weaponized for social control by authoritarian regimes, or used like the oppressive eugenics of the early 20th century. Fortunately, the realities are far more mundane and such dystopian visions are fantastical. So, what are some actual possibilities?
Some researchers suggest that neurotechnologies could help to reactivate brain regions of those suffering from moral pathologies, including healthy people with psychopathic traits (like a lack of empathy). Another proposal is using such technology on young people with conduct problems to prevent serious disorders in adulthood.
Most of us aren’t always as ethical as we would like – given the option of ‘priming’ yourself to act in consistent accord with your higher values, would you take it?
A question is whether these kinds of interventions should be compulsory for dangerous criminals. On the other hand, a voluntary treatment for inmates wouldn’t be so different from existing incentive schemes. For instance, some U.S. jurisdictions already offer drug treatment programs in exchange for early release or instead of prison time. Then there’s the difficult question of how we should treat non-criminal but potentially harmful ‘successful’ psychopaths.
Others argue that if virtues have a genetic component, there is no technological reason why present practices of embryo screening for genetic diseases couldn’t also be used for selecting socially beneficial traits.
Perhaps the most immediate scenario is a kind of voluntary moral therapy, which would use biomedicine to facilitate ideal brain-states to augment traditional psychotherapy. Most of us aren’t always as ethical as we would like – given the option of ‘priming’ yourself to act in consistent accord with your higher values, would you take it? Approaches like neurofeedback and psychedelic-assisted therapy could prove helpful.
What are the challenges?
A general challenge is that of setting. Morality is context dependent; what’s good in one environment may be bad in another and vice versa, so we don’t want to throw out the baby with the bathwater. Of course, common sense tells us that some tendencies are more socially desirable than others: fairness, altruism, and openness are clearly preferred over aggression, dishonesty, and prejudice.
One argument is that remoulding ‘brute impulses’ via biology would not count as moral enhancement. This view claims that for an action to truly count as moral it must involve cognition – reasoning, deliberation, judgement – as a necessary part of moral behavior. Critics argue that we should be concerned more with ends rather than means, so ultimately it’s outcomes that matter most.
Another worry is that modifying one biological aspect will have adverse knock-on effects for other valuable traits. Certainly, we must be careful about the network impacts of any intervention. But all stimuli have distributed effects on the body, so it’s really a matter of weighing up the cost/benefit trade-offs as in any standard medical decision.
Is it ethical?
Our values form a big part of who we are – some bioethicists argue that altering morality would pose a threat to character and personal identity. Another claim is that moral enhancement would compromise autonomy by limiting a person’s range of choices and curbing their ‘freedom to fall.’ Any intervention must consider the potential impacts on selfhood and personal liberty, in addition to the wider social implications.
This includes the importance of social and genetic diversity, which is closely tied to considerations of fairness, equality, and opportunity. The history of psychiatry is rife with examples of systematic oppression, like ‘drapetomania’ – the spurious mental illness that was thought to cause African slaves’ desire to flee captivity. Advocates for using moral enhancement technologies to help kids with conduct problems should be mindful that they disproportionately come from low-income communities. We must ensure that any habilitative practice doesn’t perpetuate harmful prejudices by unfairly targeting marginalized people.
Human capacities are the result of environmental influences, and external conditions still coax our biology in unknown ways. Status quo bias for ‘letting nature take its course’ may actually be worse long term – failing to utilize technology for human development may do more harm than good.
Then, there are concerns that morally-enhanced persons would be vulnerable to predation by those who deliberately avoid moral therapies. This relates to what’s been dubbed the ‘bootstrapping problem’: would-be moral enhancement candidates are the types of individuals that benefit from not being morally enhanced. Imagine if every senator was asked to undergo an honesty-boosting procedure prior to entering public office – would they go willingly? Then again, perhaps a technological truth-serum wouldn’t be such a bad requisite for those in positions of stern social consequence.
Advocates argue that biomedical moral betterment would simply offer another means of pursuing the same goals as fixed social mechanisms like religion, education, and community, and non-invasive therapies like cognitive-behavior therapy and meditation. It’s even possible that technological efforts would be more effective. After all, human capacities are the result of environmental influences, and external conditions still coax our biology in unknown ways. Status quo bias for ‘letting nature take its course’ may actually be worse long term – failing to utilize technology for human development may do more harm than good. If we can safely improve ourselves in direct and deliberate ways then there’s no morally significant difference whether this happens via conventional methods or new technology.
Future prospects
Where speculation about human enhancement has led to hype and technophilia, many bioethicists urge restraint. We can be grounded in current science while anticipating feasible medium-term prospects. It’s unlikely moral enhancement heralds any metamorphic post-human utopia (or dystopia), but that doesn’t mean dismissing its transformative potential. In one sense, we should be wary of transhumanist fervour about the salvatory promise of new technology. By the same token we must resist technofear and alarmist efforts to balk social and scientific progress. Emerging methods will continue to shape morality in subtle and not-so-subtle ways – the critical steps are spotting and scaffolding these with robust ethical discussion, public engagement, and reasonable policy options. Steering a bright and judicious course requires that we pilot the possibilities of morally-disruptive technologies.
Podcast: The Friday Five - your health research roundup
The Friday Five is a new series in which Leaps.org covers five breakthroughs in research over the previous week that you may have missed.
The Friday Five is a new podcast series in which Leaps.org covers five breakthroughs in research over the previous week that you may have missed. There are plenty of controversies and ethical issues in science – and we get into many of them in our online magazine – but there’s also plenty to be excited about, and this news roundup is focused on inspiring scientific work to give you some momentum headed into the weekend.
Covered in this week's Friday Five:
- Puffer fish chemical for treating chronic pain
- Sleep study on the health benefits of waking up multiples times per night
- Best exercise regimens for reducing the risk of mortality aka living longer
- AI breakthrough in mapping protein structures with DeepMind
- Ultrasound stickers to see inside your body

