Is Finding Out Your Baby’s Genetics A New Responsibility of Parenting?
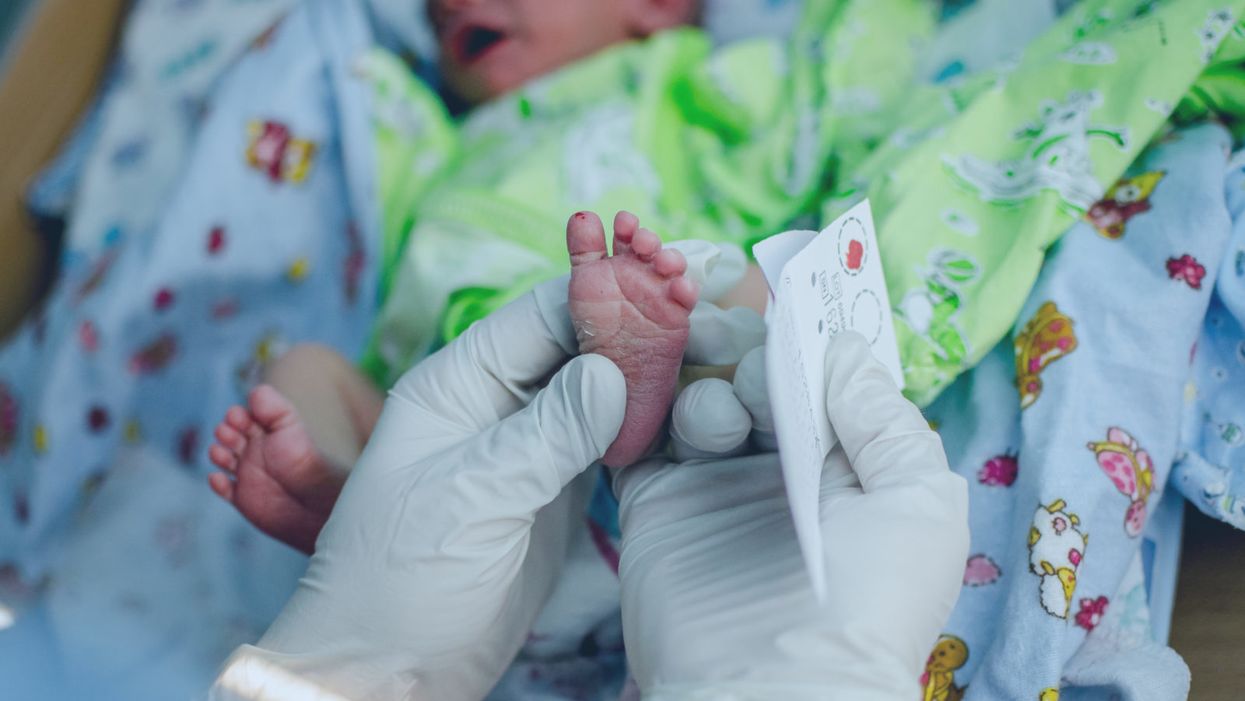
A doctor pricks the heel of a newborn for a blood test.
Hours after a baby is born, its heel is pricked with a lancet. Drops of the infant's blood are collected on a porous card, which is then mailed to a state laboratory. The dried blood spots are screened for around thirty conditions, including phenylketonuria (PKU), the metabolic disorder that kick-started this kind of newborn screening over 60 years ago. In the U.S., parents are not asked for permission to screen their child. Newborn screening programs are public health programs, and the assumption is that no good parent would refuse a screening test that could identify a serious yet treatable condition in their baby.
Learning as much as you can about your child's health might seem like a natural obligation of parenting. But it's an assumption that I think needs to be much more closely examined.
Today, with the introduction of genome sequencing into clinical medicine, some are asking whether newborn screening goes far enough. As the cost of sequencing falls, should parents take a more expansive look at their children's health, learning not just whether they have a rare but treatable childhood condition, but also whether they are at risk for untreatable conditions or for diseases that, if they occur at all, will strike only in adulthood? Should genome sequencing be a part of every newborn's care?
It's an idea that appeals to Anne Wojcicki, the founder and CEO of the direct-to-consumer genetic testing company 23andMe, who in a 2016 interview with The Guardian newspaper predicted that having newborns tested would soon be considered standard practice—"as critical as testing your cholesterol"—and a new responsibility of parenting. Wojcicki isn't the only one excited to see everyone's genes examined at birth. Francis Collins, director of the National Institutes of Health and perhaps the most prominent advocate of genomics in the United States, has written that he is "almost certain … that whole-genome sequencing will become part of new-born screening in the next few years." Whether that would happen through state-mandated screening programs, or as part of routine pediatric care—or perhaps as a direct-to-consumer service that parents purchase at birth or receive as a baby-shower gift—is not clear.
Learning as much as you can about your child's health might seem like a natural obligation of parenting. But it's an assumption that I think needs to be much more closely examined, both because the results that genome sequencing can return are more complex and more uncertain than one might expect, and because parents are not actually responsible for their child's lifelong health and well-being.
What is a parent supposed to do about such a risk except worry?
Existing newborn screening tests look for the presence of rare conditions that, if identified early in life, before the child shows any symptoms, can be effectively treated. Sequencing could identify many of these same kinds of conditions (and it might be a good tool if it could be targeted to those conditions alone), but it would also identify gene variants that confer an increased risk rather than a certainty of disease. Occasionally that increased risk will be significant. About 12 percent of women in the general population will develop breast cancer during their lives, while those who have a harmful BRCA1 or BRCA2 gene variant have around a 70 percent chance of developing the disease. But for many—perhaps most—conditions, the increased risk associated with a particular gene variant will be very small. Researchers have identified over 600 genes that appear to be associated with schizophrenia, for example, but any one of those confers only a tiny increase in risk for the disorder. What is a parent supposed to do about such a risk except worry?
Sequencing results are uncertain in other important ways as well. While we now have the ability to map the genome—to create a read-out of the pairs of genetic letters that make up a person's DNA—we are still learning what most of it means for a person's health and well-being. Researchers even have a name for gene variants they think might be associated with a disease or disorder, but for which they don't have enough evidence to be sure. They are called "variants of unknown (or uncertain) significance (VUS), and they pop up in most people's sequencing results. In cancer genetics, where much research has been done, about 1 in 5 gene variants are reclassified over time. Most are downgraded, which means that a good number of VUS are eventually designated benign.
While one parent might reasonably decide to learn about their child's risk for a condition about which nothing can be done medically, a different, yet still thoroughly reasonable, parent might prefer to remain ignorant so that they can enjoy the time before their child is afflicted.
Then there's the puzzle of what to do about results that show increased risk or even certainty for a condition that we have no idea how to prevent. Some genomics advocates argue that even if a result is not "medically actionable," it might have "personal utility" because it allows parents to plan for their child's future needs, to enroll them in research, or to connect with other families whose children carry the same genetic marker.
Finding a certain gene variant in one child might inform parents' decisions about whether to have another—and if they do, about whether to use reproductive technologies or prenatal testing to select against that variant in a future child. I have no doubt that for some parents these personal utility arguments are persuasive, but notice how far we've now strayed from the serious yet treatable conditions that motivated governments to set up newborn screening programs, and to mandate such testing for all.
Which brings me to the other problem with the call for sequencing newborn babies: the idea that even if it's not what the law requires, it's what good parents should do. That idea is very compelling when we're talking about sequencing results that show a serious threat to the child's health, especially when interventions are available to prevent or treat that condition. But as I have shown, many sequencing results are not of this type.
While one parent might reasonably decide to learn about their child's risk for a condition about which nothing can be done medically, a different, yet still thoroughly reasonable, parent might prefer to remain ignorant so that they can enjoy the time before their child is afflicted. This parent might decide that the worry—and the hypervigilence it could inspire in them—is not in their child's best interest, or indeed in their own. This parent might also think that it should be up to the child, when he or she is older, to decide whether to learn about his or her risk for adult-onset conditions, especially given that many adults at high familial risk for conditions like Alzheimer's or Huntington's disease choose never to be tested. This parent will value the child's future autonomy and right not to know more than they value the chance to prepare for a health risk that won't strike the child until 40 or 50 years in the future.
Parents are not obligated to learn about their children's risk for a condition that cannot be prevented, has a small risk of occurring, or that would appear only in adulthood.
Contemporary understandings of parenting are famously demanding. We are asked to do everything within our power to advance our children's health and well-being—to act always in our children's best interests. Against that backdrop, the need to sequence every newborn baby's genome might seem obvious. But we should be skeptical. Many sequencing results are complex and uncertain. Parents are not obligated to learn about their children's risk for a condition that cannot be prevented, has a small risk of occurring, or that would appear only in adulthood. To suggest otherwise is to stretch parental responsibilities beyond the realm of childhood and beyond factors that parents can control.
Scientists Are Studying How to Help Dogs Have Longer Lives, in a Bid to Further Our Own
Feeding dogs only once a day is showing health benefits in a large study, scientists report.
The sad eyes. The wagging tail. The frustrated whine. The excited bark. Dogs know how to get their owners to fork over the food more often.
The extra calories dogs get from feeding patterns now used by many Americans may not be good for them from a health and longevity viewpoint. In research from a large study called the Dog Aging Project, canines fed once a day had better scores on cognition tests and lower odds of developing diseases of organs throughout the body: intestinal tract, mouth and teeth, bones and joints, kidneys and bladder, and liver and pancreas.
Fewer than 1 in 10 dog owners fed their furry friends once daily, while nearly three fourths provided two daily meals.
“Most veterinarians have been led to believe that feeding dogs twice a day is optimal, but this is a relatively new idea that has developed over the past few decades with little supportive evidence from a health standpoint,” said Matt Kaeberlein, PhD, Co-Director of the Dog Aging Project, a professor of pathology and Director of the Healthy Aging and Longevity Research Institute at the University of Washington. Kaeberlein studies basic mechanisms of aging to find ways of extending the healthspan, the number of years of life lived free of disease. It’s not enough to extend the lifespan unless declines in biological function and risks of age-related diseases are also studied, he believes, hence the healthspan.
The Dog Aging Project is studying tens of thousands of dogs living with their owners in the real world, not a biology laboratory. The feeding study is the first of several reports now coming from the project based on owners’ annual reports of demographics, physical activity, environment, dog behavior, diet, medications and supplements, and health status. It has been posted on bioRxiv as it goes through peer review.
“All available evidence suggests that most biological mechanisms of aging in dogs will be conserved in humans. It just happens much faster in dogs.”
“The Dog Aging Project is one of the most exciting in the longevity space,” said David A. Sinclair, professor in the Department of Genetics and co-director of the Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School. “Not only is it important to help our companions live longer and healthier, but because they are like people and share the same environment and many of the lifestyles as their owners, they are the perfect model for human longevity interventions.”
The epigenetic clock — and specifically changes in gene expression resulting from methylation of cytosine and guanine in the DNA — provides the critical connection between aging in dogs and people. “All available evidence suggests that most biological mechanisms of aging in dogs will be conserved in humans,” Kaeberlein said. “It just happens much faster in dogs.” These methylation changes, called the “methylomes,” have been associated with rates of aging in dogs, humans, and also mice.
In a 2020 study young dogs matched with young adults and aged dogs matched with older adults showed the greatest similarities in methylomes. In the Cell Systems report, Tina Wang of the University of California, San Diego, and colleagues wrote that the methylome “can be used to quantitatively translate the age-related physiology experienced by one organism (i.e., a model species like dog) to the age at which physiology in a second organism is most similar (i.e., a second model or humans).” This allows rates of aging in one species to be mapped onto aging in another species, providing “a compelling tool in the quest to understand aging and identify interventions for maximizing healthy lifespan.”
In the Dog Aging Project study, 8% of 24,238 owners fed their dogs once daily, the same as the percentage of owners serving three daily meals. Twice-daily feedings were most common (73%), and just over 1 in 10 owners (11%) “free fed” their dogs by just filling up the bowl whenever it was empty — most likely Rover’s favorite option.
“The notion of breakfast, lunch, and dinner for people in the United States is not based on large studies that compared three meals a day to two meals a day, or to four, “ said Kate E. Creevy, chief veterinary officer with the Dog Aging Project and associate professor at Texas A&M University. “It’s more about what we are accustomed to. Similarly, there are not large population studies comparing outcomes of dogs fed once, twice, or three times a day.”
“We do not recommend that people change their dogs’ diets based on this report,” Creevy emphasized. “It’s important to understand the difference between research that finds associations versus research that finds cause and effect.”
To establish cause and effect, the Dog Aging Project will follow their cohort over many years. Then, Creevy said, “We will be able to determine whether the associations we have found with feeding frequency are causes, or effects, or neither.”
While not yet actionable, the feeding findings fit with biology across a variety of animals, Kaeberlein said, including indicators that better health translates into longer healthspans. He said that caloric restriction and perhaps time-restricted eating or intermittent fasting — all ways that some human diets are structured — can have a positive impact on the biology of aging by allowing the gastrointestinal tract to have time each day to rest and repair itself, just as sleep benefits the brain through rest.
Timing of meals is also related to the concept of ketogenesis, Kaeberlein explained. Without access to glucose, animals switch over to a ketogenic state in which back-up systems produce energy through metabolic pathways that generate ketones. Mice go into this state very quickly, after a few hours or an overnight fast, while people shift to ketogenesis more slowly, from a few hours to up to 36 hours for people on typical Western diets, Kaeberlein said.
Dogs are different. They take at least two days to shift to ketogenesis, suggesting they have evolved to need fewer meals that are spaced out rather than the multiple daily meals plus snacks that people prefer.
As this relates to longevity, Kaeberlein said that a couple of studies show that mice who are fed a ketogenic diet have longer lifespans (years of life regardless of health). “For us, the next step is to analyze the composition of the dogs’ diets or the relationship of multiple daily feedings with obesity,” he said. “Maybe not being obese is related to better health.”
To learn more, the Dog Aging Project needs dogs — lots of dogs! Kaeberlein wants at least 100,000 dogs, including small dogs, large dogs, dogs of all ages. Puppies are needed for the researchers to follow across their lifespan. The project has an excellent website where owners can volunteer to participate.
Nutritional strategies are often not built around sound scientific principles, Kaeberlein said. In human nutrition, people have tried all kinds of diets over the years, including some that were completely wrong. Kaeberlein and his colleagues in the Dog Aging Project want to change that, at least for people’s canine companions, and hopefully, as a result, give dogs added years of healthy life and provide clues for human nutrition.
After that, maybe they can do something about those sad eyes and the frustrated whine.
Podcast: New Solutions to Combat Gluten Sensitivities and Food Allergies
Biotech company Ukko is designing proteins that will be safe for everyone to eat, starting with peanut and gluten.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
This month, we talk Anat Binur, the CEO of Israeli/U.S.-based biotech company Ukko. Ukko is taking a revolutionary approach to the distressing problem of food allergies and gluten sensitivities: their scientists are designing and engineering proteins that keep the good biophysical properties of the original proteins, while removing the immune-triggering parts that can cause life-threatening allergies. The end goal is proteins that are safe for everyone. Ukko is focusing first on developing a new safe gluten protein for use in baking and a new peanut protein for use as a therapeutic. Their unique platform could theoretically be used for any protein-based allergy, including cats and bees. Hear more in this episode.
Watch the 60-second trailer
Listen to the whole episode
<div id="buzzsprout-player-9950980"></div><script src="https://www.buzzsprout.com/1714953/9950980-solving-food-allergies-with-biotech-company-ukko.js?container_id=buzzsprout-player-9950980&player=small" type="text/javascript" charset="utf-8"></script>
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

