Just Say No to Editing Human Embryos for Reproduction
Insemination of female egg under microscope
BIG QUESTION OF THE MONTH: Should we use CRISPR, the new technique that enables precise DNA editing, to change the genes of human embryos to eradicate disease – or even to enhance desirable traits? LeapsMag invited three leading experts to weigh in.
Over the last few decades, the international community has issued several bioethical guidelines and legally binding documents, ranging from UN Declarations to regional charters to national legislation, about editing the human germline--the DNA that is passed down to future generations. There was a broad consensus that modifications should be prohibited. But now that CRISPR-cas9 and related methods of gene editing are taking the world by storm, that stance is softening--and so far, no thorough public discussion has emerged.
There is broad agreement in the scientific and ethics community that germline gene editing must not be clinically applied unless safety concerns are resolved. Predicting that safety issues will indeed be minimized, the National Academy of Sciences issued a report this past February that sets up several procedural norms. These may serve as guidelines for future implementation of human embryo editing, among them that there are no "reasonable alternatives," a condition that is left deliberately vague.
I regard the conditional embrace of germline gene editing as a grave mistake: It is a dramatic break with the previous idea of a ban, departing also from the moratorium that the UNESCO International Bioethics Committee had recommended in 2015. But in a startling move, the Academy already set the next post, recommending "that genome editing for purposes other than treatment or prevention of disease and disability should not proceed at this time" (my emphasis). It recommended public discussions, but without spelling out its own role in facilitating them.
"The international community should explicitly ban embryo gene editing as a method of human reproduction."
To proceed ethically, I argue that the international community, through the United Nations and in line with the ban on human reproductive cloning, should explicitly ban embryo gene editing as a method of human reproduction. Together with guidelines adjusted for non-reproductive and non-human applications, a prohibition would ensure two important results: First, that non-reproductive human embryo research could be pursued in a responsible way in those countries that allow for it, and second, that individual scientists, public research institutes, and private companies would know the moral limit of possible research.
Basic human embryo research is required, scientists argue, to better understand genetic diseases and early human development. I do not question this, and I am convinced that existing guidelines can be adjusted to meet the moral requirements in this area. Millions of people may benefit from different non-reproductive pathways of gene editing. Germline gene editing, in contrast, does not offer any resolutions to global or local health problems – and that alone raises many concerns about the current state of scientific research.
I support a ban because germline gene editing for reproductive purposes concerns more than safety. The genetic modification of a human being is irreversible and unpredictable in its epigenetic, personal, and social effects. It concerns the rights of children; it exposes persons with disabilities to social stigmatization; it contradicts the global justice agenda with respect to healthcare; and it infringes upon the rights to freedom and well-being of future persons.
"Reproductive germline gene editing directly violates the rights of individual future person."
Apart from questions of justice, reproductive germline gene editing may well increase the stigmatization of persons with disabilities. I want to emphasize here, however, that it directly violates the rights of individual future persons, namely a future child's right to genetic integrity, to freedom, and potentially to well-being, all guaranteed in different UN Declarations of Human Rights. For all these reasons, it is an unacceptable path forward.
The way the discussion has been framed so far is very different from my perspective that situates germline gene editing in the broader framework of human rights and responsibilities. In short, many others never questioned the goal but instead focused on the unintentional side-effects of an otherwise beneficial technique for human reproduction. Some scientists see germline gene editing as an alternative to embryo selection via Preimplantation Genetic Diagnosis (PGD), a procedure in which multiple embryos are tested to find out which ones carry disease-causing mutations. Others see it as the first step to human enhancement.
Some physicians argue that in the field of assisted reproduction, not every couple is comfortable with embryo selection via PGD, because potentially, unchosen embryos are discarded. Germline gene editing offers them an alternative. It is rarely mentioned, however, that germline gene editing would most likely still require PGD as a control of the procedure (though without the purpose of selection), and that prenatal genetic diagnosis would also be highly recommended. In other words, germline gene editing would not replace existing protocols but rather change their purpose, and it would also not necessarily reduce the number of embryos needed for assisted reproduction.
In some (rare) cases, PGD is not an option, because in the couples' condition, all embryos will be affected. One current option to avoid transmitting genetic traits is to use a donor sperm or egg, though the resulting child would not be genetically related to one parent. If these parents had an obligation, as some proponents argue, to secure the health of their offspring (an argument that I do not follow), then procreation with sperm or egg donation would even be morally required, as this is the safest procedure to erase a given genetic trait.
There are no therapeutic scenarios that exclusively require reproductive gene editing even if one accepts the right to reproductive autonomy. The fact is that couples who rightly wish to secure and protect the health of their future children can be offered medical alternatives in all cases. However, this requires considering sperm or egg donation as the safest and most reasonable option – the condition the NAS Report has set.
Scientists in favor of germline gene editing argue against this: the desire for genetic kinship, they say, is a legitimate expression of a couple's reproductive freedom, and germline gene editing offers them an alternative to have a healthy child. In the future, proponents say, these (very few) couples who wish for genetically related offspring will be faced with the dilemma of either accepting the transmission of a genetic health risk to their children or weighing the benefits and risks of gene editing.
But here is a blind spot in the whole discussion.
Many scientists and some bioethicists think that reproductive freedom includes the right to a genetically related child. But even if we were to presuppose such a right, it is not absolute in the context of assisted reproduction. Although sperm or egg donation may be undesirable for some couples, the moral question of responsibility does not disappear with their reproductive rights. At a minimum, the future child's rights must be considered, and these rights go further than their health rights.
It is puzzling that in claiming their own reproductive freedom, couples would need to ignore their children's and possibly grandchildren's future freedom – including the constraints resulting from being monitored over the course of their lives and the indirect constraints of the children's own right to reproductive freedom. From a medical standpoint, it would be highly recommended for them, too, to have children through assisted reproduction. This distinguishes germline gene editing from any other procedure of assisted reproduction: we need the data from the second and third generations to see whether the method is safe and efficacious. Whose reproductive freedom should count, the parents' or the future children's?
But for now, the question of parental rights may well divert the discussion from the question of responsible gene editing research; its conditions and structures require urgent evaluation and adjustment to guide international research groups. I am concerned that we are in the process of developing a new technology that has tremendous potential and ramifications – but without having considered the ethical framework for a responsible path forward.
Editor's Note: Check out the viewpoints expressing enthusiastic support and mild curiosity.
Dr. May Edward Chinn, Kizzmekia Corbett, PhD., and Alice Ball, among others, have been behind some of the most important scientific work of the last century.
If you look back on the last century of scientific achievements, you might notice that most of the scientists we celebrate are overwhelmingly white, while scientists of color take a backseat. Since the Nobel Prize was introduced in 1901, for example, no black scientists have landed this prestigious award.
The work of black women scientists has gone unrecognized in particular. Their work uncredited and often stolen, black women have nevertheless contributed to some of the most important advancements of the last 100 years, from the polio vaccine to GPS.
Here are five black women who have changed science forever.
Dr. May Edward Chinn
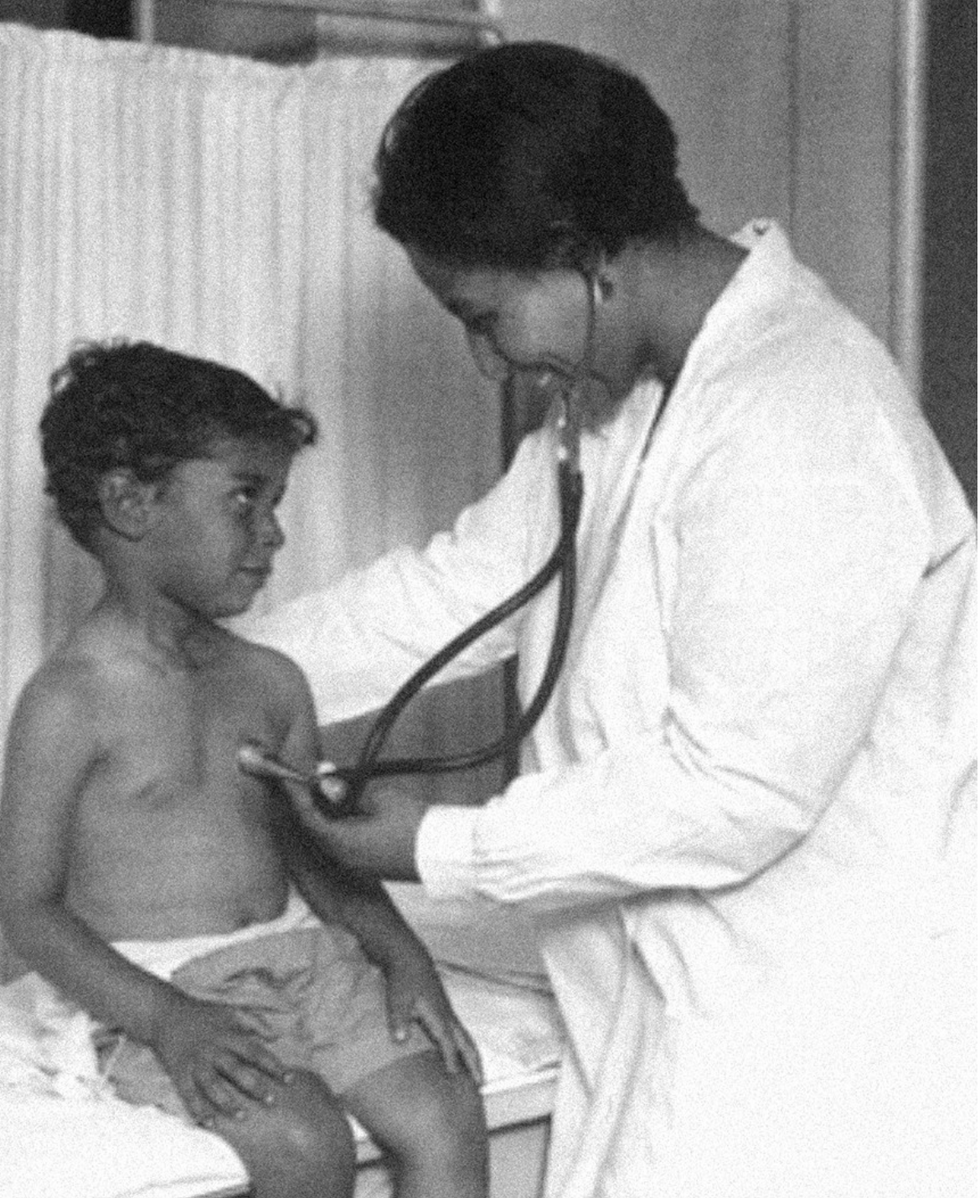
Dr. May Edward Chinn practicing medicine in Harlem
George B. Davis, PhD.
Chinn was born to poor parents in New York City just before the start of the 20th century. Although she showed great promise as a pianist, playing with the legendary musician Paul Robeson throughout the 1920s, she decided to study medicine instead. Chinn, like other black doctors of the time, were barred from studying or practicing in New York hospitals. So Chinn formed a private practice and made house calls, sometimes operating in patients’ living rooms, using an ironing board as a makeshift operating table.
Chinn worked among the city’s poor, and in doing this, started to notice her patients had late-stage cancers that often had gone undetected or untreated for years. To learn more about cancer and its prevention, Chinn begged information off white doctors who were willing to share with her, and even accompanied her patients to other clinic appointments in the city, claiming to be the family physician. Chinn took this information and integrated it into her own practice, creating guidelines for early cancer detection that were revolutionary at the time—for instance, checking patient health histories, checking family histories, performing routine pap smears, and screening patients for cancer even before they showed symptoms. For years, Chinn was the only black female doctor working in Harlem, and she continued to work closely with the poor and advocate for early cancer screenings until she retired at age 81.
Alice Ball

Pictorial Press Ltd/Alamy
Alice Ball was a chemist best known for her groundbreaking work on the development of the “Ball Method,” the first successful treatment for those suffering from leprosy during the early 20th century.
In 1916, while she was an undergraduate student at the University of Hawaii, Ball studied the effects of Chaulmoogra oil in treating leprosy. This oil was a well-established therapy in Asian countries, but it had such a foul taste and led to such unpleasant side effects that many patients refused to take it.
So Ball developed a method to isolate and extract the active compounds from Chaulmoogra oil to create an injectable medicine. This marked a significant breakthrough in leprosy treatment and became the standard of care for several decades afterward.
Unfortunately, Ball died before she could publish her results, and credit for this discovery was given to another scientist. One of her colleagues, however, was able to properly credit her in a publication in 1922.
Henrietta Lacks

onathan Newton/The Washington Post/Getty
The person who arguably contributed the most to scientific research in the last century, surprisingly, wasn’t even a scientist. Henrietta Lacks was a tobacco farmer and mother of five children who lived in Maryland during the 1940s. In 1951, Lacks visited Johns Hopkins Hospital where doctors found a cancerous tumor on her cervix. Before treating the tumor, the doctor who examined Lacks clipped two small samples of tissue from Lacks’ cervix without her knowledge or consent—something unthinkable today thanks to informed consent practices, but commonplace back then.
As Lacks underwent treatment for her cancer, her tissue samples made their way to the desk of George Otto Gey, a cancer researcher at Johns Hopkins. He noticed that unlike the other cell cultures that came into his lab, Lacks’ cells grew and multiplied instead of dying out. Lacks’ cells were “immortal,” meaning that because of a genetic defect, they were able to reproduce indefinitely as long as certain conditions were kept stable inside the lab.
Gey started shipping Lacks’ cells to other researchers across the globe, and scientists were thrilled to have an unlimited amount of sturdy human cells with which to experiment. Long after Lacks died of cervical cancer in 1951, her cells continued to multiply and scientists continued to use them to develop cancer treatments, to learn more about HIV/AIDS, to pioneer fertility treatments like in vitro fertilization, and to develop the polio vaccine. To this day, Lacks’ cells have saved an estimated 10 million lives, and her family is beginning to get the compensation and recognition that Henrietta deserved.
Dr. Gladys West

Andre West
Gladys West was a mathematician who helped invent something nearly everyone uses today. West started her career in the 1950s at the Naval Surface Warfare Center Dahlgren Division in Virginia, and took data from satellites to create a mathematical model of the Earth’s shape and gravitational field. This important work would lay the groundwork for the technology that would later become the Global Positioning System, or GPS. West’s work was not widely recognized until she was honored by the US Air Force in 2018.
Dr. Kizzmekia "Kizzy" Corbett

TIME Magazine
At just 35 years old, immunologist Kizzmekia “Kizzy” Corbett has already made history. A viral immunologist by training, Corbett studied coronaviruses at the National Institutes of Health (NIH) and researched possible vaccines for coronaviruses such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome).
At the start of the COVID pandemic, Corbett and her team at the NIH partnered with pharmaceutical giant Moderna to develop an mRNA-based vaccine against the virus. Corbett’s previous work with mRNA and coronaviruses was vital in developing the vaccine, which became one of the first to be authorized for emergency use in the United States. The vaccine, along with others, is responsible for saving an estimated 14 million lives.On today’s episode of Making Sense of Science, I’m honored to be joined by Dr. Paul Song, a physician, oncologist, progressive activist and biotech chief medical officer. Through his company, NKGen Biotech, Dr. Song is leveraging the power of patients’ own immune systems by supercharging the body’s natural killer cells to make new treatments for Alzheimer’s and cancer.
Whereas other treatments for Alzheimer’s focus directly on reducing the build-up of proteins in the brain such as amyloid and tau in patients will mild cognitive impairment, NKGen is seeking to help patients that much of the rest of the medical community has written off as hopeless cases, those with late stage Alzheimer’s. And in small studies, NKGen has shown remarkable results, even improvement in the symptoms of people with these very progressed forms of Alzheimer’s, above and beyond slowing down the disease.
In the realm of cancer, Dr. Song is similarly setting his sights on another group of patients for whom treatment options are few and far between: people with solid tumors. Whereas some gradual progress has been made in treating blood cancers such as certain leukemias in past few decades, solid tumors have been even more of a challenge. But Dr. Song’s approach of using natural killer cells to treat solid tumors is promising. You may have heard of CAR-T, which uses genetic engineering to introduce cells into the body that have a particular function to help treat a disease. NKGen focuses on other means to enhance the 40 plus receptors of natural killer cells, making them more receptive and sensitive to picking out cancer cells.
Paul Y. Song, MD is currently CEO and Vice Chairman of NKGen Biotech. Dr. Song’s last clinical role was Asst. Professor at the Samuel Oschin Cancer Center at Cedars Sinai Medical Center.
Dr. Song served as the very first visiting fellow on healthcare policy in the California Department of Insurance in 2013. He is currently on the advisory board of the Pritzker School of Molecular Engineering at the University of Chicago and a board member of Mercy Corps, The Center for Health and Democracy, and Gideon’s Promise.
Dr. Song graduated with honors from the University of Chicago and received his MD from George Washington University. He completed his residency in radiation oncology at the University of Chicago where he served as Chief Resident and did a brachytherapy fellowship at the Institute Gustave Roussy in Villejuif, France. He was also awarded an ASTRO research fellowship in 1995 for his research in radiation inducible gene therapy.
With Dr. Song’s leadership, NKGen Biotech’s work on natural killer cells represents cutting-edge science leading to key findings and important pieces of the puzzle for treating two of humanity’s most intractable diseases.
Show links
- Paul Song LinkedIn
- NKGen Biotech on Twitter - @NKGenBiotech
- NKGen Website: https://nkgenbiotech.com/
- NKGen appoints Paul Song
- Patient Story: https://pix11.com/news/local-news/long-island/promising-new-treatment-for-advanced-alzheimers-patients/
- FDA Clearance: https://nkgenbiotech.com/nkgen-biotech-receives-ind-clearance-from-fda-for-snk02-allogeneic-natural-killer-cell-therapy-for-solid-tumors/Q3 earnings data: https://www.nasdaq.com/press-release/nkgen-biotech-inc.-reports-third-quarter-2023-financial-results-and-business