This Resistance Fighter Invented Dialysis in Nazi-Occupied Holland
When Willem Johan Kolff invented dialysis, the "father" of artificial organs was just getting started.
One of the Netherlands’ most famous pieces of pop culture is “Soldier of Orange.” It’s the title of the country’s most celebrated war memoir, movie and epic stage musical, all of which detail the exploits of the nation’s resistance fighters during World War II.
Willem Johan Kolff was a member of the Dutch resistance, but he doesn’t rate a mention in the “Solider of Orange” canon. Yet his wartime toils in a rural backwater not only changed medicine, but the world.
Kolff had been a physician less than two years before Germany invaded the Netherlands in May 1940. He had been engaged in post-graduate studies at the University of Gronigen but withdrew because he refused to accommodate the demands of the Nazi occupiers. Kolff’s Jewish supervisor made an even starker choice: He committed suicide.
After his departure from the university, Kolff took a job managing a small hospital in Kampen. Located 50 miles from the heavily populated coastal region, the facility was far enough away from the prying eyes of Germans that not only could Kolff care for patients, he could hide fellow resistance fighters and even Jewish refugees in relative safety. Kolff coached many of them to feign convincing terminal illnesses so the Nazis would allow them to remain in the hospital.
Despite the demands of practicing medicine and resistance work, Kolff still found time to conduct research. He had been haunted and inspired when, not long before the Nazi invasion, one of his patients died in agony from kidney disease. Kolff wanted to find a way to save future patients.
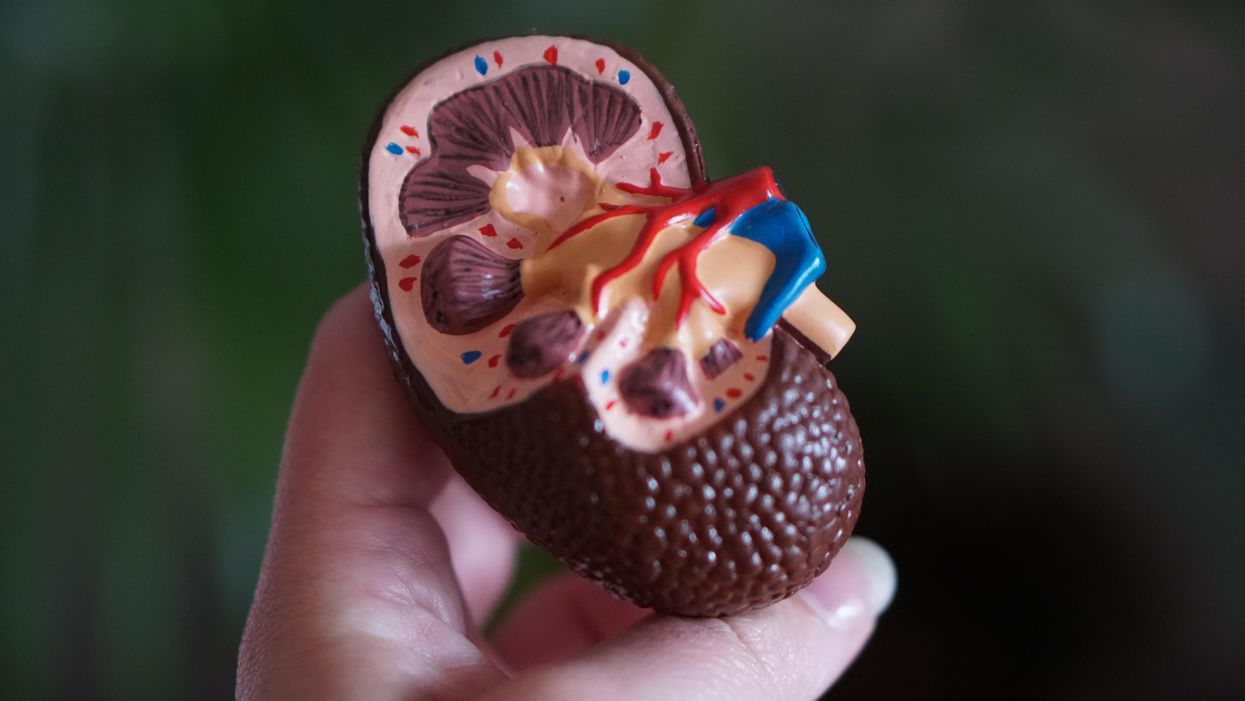
He broke his problem down to a simple task: If he could remove 20 grams of urea from a patient’s blood in 24 hours, they would survive. He began experimenting with ways to filter blood and return it to a patient’s body. Since the war had ground all non-military manufacturing to a halt, he was mostly forced to make do with material he could find at the hospital and around Kampen. Kolff eventually built a device from a washing machine parts, juice cans, sausage casings, a valve from an old Ford automobile radiator, and even scrap from a downed German aircraft.
The world’s first dialysis machine was hardly imposing; it resembled a rotating drum for a bingo game or raffle. Yet it carried on the highly sophisticated task of moving a patient’s blood through a semi-permeable membrane (about a 50-foot length of sausage casings) into a saline solution that drew out urea while leaving the blood cells untouched.
In emigrating to the U.S. to practice medicine, Kolff's intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
Kolff began using the machine to treat patients in 1943, most of whom had lapsed into comas due to their kidney failure. But like most groundbreaking medical devices, it was not an immediate success. By the end of the war, Kolff had dialyzed more than a dozen patients, but all had died. He briefly suspended use of the device after the Allied invasion of Europe, but he continued to refine its operation and the administration of blood thinners to patients.
In September 1945, Kolff dialyzed another comatose patient, 67-year-old Sofia Maria Schafstadt. She regained consciousness after 11 hours, and would live well into the 1950s with Kolff’s assistance. Yet this triumph contained a dark irony: At the time of her treatment, Schafstadt had been imprisoned for collaborating with the Germans.
With a tattered Europe struggling to overcome the destruction of the war, Kolff and his family emigrated to the U.S. in 1950, where he began working for the Cleveland Clinic while undergoing the naturalization process so he could practice medicine in the U.S. His intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
By the mid-1950s, dialysis machines had become reliable and life-saving medical devices, and Kolff had become a U.S. citizen. About that time he invented a membrane oxygenator that could be used in heart bypass surgeries. This was a critical component of the heart-lung machine, which would make heart transplants possible and bypass surgeries routine. He also invented among the very first practical artificial hearts, which in 1957 kept a dog alive for 90 minutes.
Kolff moved to the University of Utah in 1967 to become director of its Institute for Biomedical Engineering. It was a promising time for such a move, as the first successful transplant of a donor heart to a human occurred that year. But he was interested in going a step further and creating an artificial heart for human use.
It took more than a decade of tinkering and research, but in 1982, a team of physicians and engineers led by Kolff succeeded in implanting the first artificial heart in dentist Barney Clark, whose failing health disqualified him from a heart transplant. Although Clark died in March 1983 after 112 days tethered to the device, that it kept him alive generated international headlines. While graduate student Robert Jarvik received the named credit for the heart, he was directly supervised by Kolff, whose various endeavors into artificial organ research at the University of Utah were segmented into numerous teams.
Forty years later, several artificial hearts have been approved for use by the Food and Drug Administration, although all are a “bridge” that allow patients to wait for a transplant.
Kolff continued researching and tinkering with biomedical devices – including artificial eyes and ears – until he retired in 1997 at the age of 86. When he died in 2009, the medical community acknowledged that he was not only a pioneer in biotechnology, but the “father” of artificial organs.
Why Neglected Tropical Diseases Should Matter to Americans
Kissing bugs can carry a parasite called Trypanosoma cruzi, which causes Chagas disease.
Daisy Hernández was five years old when one of her favorite aunts was struck with a mysterious illness. Tía Dora had stayed behind in Colombia when Daisy's mother immigrated to Union City, New Jersey. A schoolteacher in her late 20s, she began suffering from fevers and abdominal pain, and her belly grew so big that people thought she was pregnant. Exploratory surgery revealed that her large intestine had swollen to ten times its normal size, and she was fitted with a colostomy bag. Doctors couldn't identify the underlying problem—but whatever it was, they said, it would likely kill her within a year or two.
Tía Dora's sisters in New Jersey—Hernández's mother and two other aunts—weren't about to let that happen. They pooled their savings and flew her to New York City, where a doctor at Columbia-Presbyterian Medical Center with a penchant for obscure ailments provided a diagnosis: Chagas disease. Transmitted by the bite of triatomine insects, commonly known as kissing bugs, Chagas is endemic in many parts of Latin America. It's caused by the parasite Trypanoma cruzi, which usually settles in the heart, where it feeds on muscle tissue. In some cases, however, it attacks the intestines or esophagus. Tía Dora belonged to that minority.
In 1980, U.S. immigration laws were more forgiving than they are today. Tía Dora was able to have surgery to remove a part of her colon, despite not being a citizen or having a green card. She eventually married a legal resident and began teaching Spanish at an elementary school. Over the next three decades, she earned a graduate degree, built a career, and was widowed. Meanwhile, Chagas continued its slow devastation. "Every couple of years, we were back in the hospital with her," Hernández recalls. "When I was in high school, she started feeling like she couldn't swallow anything. It was the parasite, destroying the muscles of her esophagus."
When Tía Dora died in 2010, at 59, her niece was among the family members at her bedside. By then, Hernández had become a journalist and fiction writer. Researching a short story about Chagas disease, she discovered that it affected an estimated 6 million people in South America, Central America, and Mexico—as well as 300,000 in the United States, most of whom were immigrants from those places. "I was shocked to learn it wasn't rare," she says. "That made me hungry to know more about this disease, and about the families grappling with it."
Hernández's curiosity led her to write The Kissing Bug, a lyrical hybrid of memoir and science reporting that was published in June. It also led her to another revelation: Chagas is not unique. It's among the many maladies that global health experts refer to as neglected tropical diseases—often-disabling illnesses that afflict 1.7 billion people worldwide, while getting notably less attention than the "big three" of HIV/AIDs, malaria, and tuberculosis. NTDs cause fewer deaths than those plagues, but they wreak untold suffering and economic loss.
Shortly before Hernández's book hit the shelves, the World Health Organization released its 2021-2030 roadmap for fighting NTDs. The plan sets targets for controlling, eliminating, or eradicating all the diseases on the WHO's list, through measures ranging from developing vaccines to improving healthcare infrastructure, sanitation, and access to clean water. Experts agree that for the campaign to succeed, leadership from wealthy nations—particularly the United States—is essential. But given the inward turn of many such countries in recent years (evidenced in movements ranging from America First to Brexit), and the continuing urgency of the COVID-19 crisis, public support is far from guaranteed.
As Hernández writes: "It is easier to forget a disease that cannot be seen." NTDs primarily affect residents of distant lands. They kill only 80,000 people a year, down from 204,000 in 1990. So why should Americans to bother to look?
Breaking the circle of poverty and disease
The World Health Organization counts 20 diseases as NTDs. Along with Chagas, they include dengue and chikungunya, which cause high fevers and agonizing pain; elephantiasis, which deforms victims' limbs and genitals; onchocerciasis, which causes blindness; schistosomiasis, which can damage the heart, lungs, brain, and genitourinary system; helminths such as roundworm and whipworm, which cause anemia, stunted growth, and cognitive disabilities; and a dozen more. Such ailments often co-occur in the same patient, exacerbating each other's effects and those of illnesses such as malaria.
NTDs may be spread by insects, animals, soil, or tainted water; they may be parasitic, bacterial, viral, or—in the case of snakebite envenoming—non-infectious. What they have in common is their longtime neglect by public health agencies and philanthropies. In part, this reflects their typically low mortality rates. But the biggest factor is undoubtedly their disempowered patient populations.
"These diseases occur in the setting of poverty, and they cause poverty, because of their chronic and debilitating effects," observes Peter Hotez, dean of the National School of Tropical Medicine at Baylor University and co-director of the Texas Children's Hospital for Vaccine Development. And historically, the everyday miseries of impoverished people have seldom been a priority for those who set the global health agenda.
That began to change about 20 years ago, when Hotez and others developed the conceptual framework for NTDs and early proposals for combating them. The WHO released its first roadmap in 2012, targeting 17 NTDs for control, elimination, or eradication by 2020. (Rabies, snakebite, and dengue were added later.) Since then, the number of people at risk for NTDs has fallen by 600 million, and 42 countries have eliminated at least one such disease. Cases of dracunculiasis—known as Guinea worm disease, for the parasite that creates painful blisters in a patient's skin—have dropped from the millions to just 27 in 2020.
Yet the battle is not over, and the COVID-19 pandemic has disrupted prevention and treatment programs around the globe.
A new direction — and longstanding obstacles
The WHO's new roadmap sets even more ambitious goals for 2030. Among them: reducing by 90 percent the number of people requiring treatment for NTDs; eliminating at least one NTD in another 100 countries; and fully eradicating dracunculiasis and yaws, a disfiguring skin infection.
The plan also places an increased focus on "country ownership," relying on nations with high incidence of NTDs to design their own plans based on local expertise. "I was so excited to see that," says Kristina Talbert-Slagle, director of the Yale College Global Health Studies program. "No one is a better expert on how to address these situations than the people who deal with it day by day."
Another fresh approach is what the roadmap calls "cross-cutting" targets. "One of the really cool things about the plan is how much it emphasizes coordination among different sectors of the health system," says Claire Standley, a faculty member at Georgetown University's Center for Global Health Science and Security. "For example, it explicitly takes into account the zoonotic nature of many neglected tropical diseases—the fact that we have to think about animal health as well as human health when we tackle NTDs."
Whether this grand vision can be realized, however, will depend largely on funding—and that, in turn, is a question of political will in the countries most able to provide it. On the upside, the U.S. has ended its Trump-era feud with the WHO. "One thing that's been really encouraging," says Standley, "has been the strong commitment toward global cooperation from the current administration." Even under the previous president, the U.S. remained the single largest contributor to the global health kitty, spending over $100 million annually on NTDs—six times the figure in 2006, when such financing started.
On the downside, America's outlay has remained flat for several years, and the Biden administration has so far not moved to increase it. A "back-of-the-envelope calculation," says Hotez, suggests that the current level of aid could buy medications for the most common NTDs for about 200 million people a year. But the number of people who need treatment, he notes, is at least 750 million.
Up to now, the United Kingdom—long the world's second-most generous health aid donor—has taken up a large portion of the slack. But the UK last month announced deep cuts in its portfolio, eliminating 102 previously supported countries and leaving only 34. "That really concerns me," Hotez says.
The struggle for funds, he notes, is always harder for projects involving NTDs than for those aimed at higher-profile diseases. His lab, which he co-directs with microbiologist Maria Elena Bottazzi, started developing a COVID-19 vaccine soon after the pandemic struck, for example, and is now in Phase 3 trials. The team has been working on vaccines for Chagas, hookworm, and schistosomiasis for much longer, but trials for those potential game-changers lag behind. "We struggle to get the level of resources needed to move quickly," Hotez explains.
Two million reasons to care
One way to prompt a government to open its pocketbook is for voters to clamor for action. A longtime challenge with NTDs, however, has been getting people outside the hardest-hit countries to pay attention.
The reasons to care, global health experts argue, go beyond compassion. "When we have high NTD burden," says Talbert-Slagle, "it can prevent economic growth, prevent innovation, lead to more political instability." That, in turn, can lead to wars and mass migration, affecting economic and political events far beyond an affected country's borders.
Like Hernández's aunt Dora, many people driven out of NTD-wracked regions wind up living elsewhere. And that points to another reason to care about these diseases: Some of your neighbors might have them. In the U.S., up to 14 million people suffer from neglected parasitic infections—including 70,000 with Chagas in California alone.
When Hernández was researching The Kissing Bug, she worried that such statistics would provide ammunition to racists and xenophobes who claim that immigrants "bring disease" or exploit overburdened healthcare systems. (This may help explain some of the stigma around NTDs, which led Tía Dora to hide her condition from most people outside her family.) But as the book makes clear, these infections know no borders; they flourish wherever large numbers of people lack access to resources that most residents of rich countries take for granted.
Indeed, far from gaming U.S. healthcare systems, millions of low-income immigrants can't access them—or must wait until they're sick enough to go to an emergency room. Since Congress changed the rules in 1996, green card holders have to wait five years before they can enroll in Medicaid. Undocumented immigrants can never qualify.
Closing the great divide
Hernández uses a phrase borrowed from global health crusader Paul Farmer to describe this access gap: "the great epi divide." On one side, she explains, "people will die from cancer, from diabetes, from chronic illnesses later in life. On the other side of the epidemiological divide, people are dying because they can't get to the doctor, or they can't get medication. They don't have a hospital anywhere near them. When I read Dr. Farmer's work, I realized how much that applied to neglected diseases as well."
When it comes to Chagas disease, she says, the epi divide is embodied in the lack of a federal mandate for prenatal or newborn screening. Each year, according to the Centers for Disease Control and Prevention, up to 300 babies in the U.S. are born with Chagas, which can be passed from the mother in utero. The disease can be cured with medication if treated in infancy. (It can also be cured in adults in the acute stage, but is seldom detected in time.) Yet the CDC does not require screening for Chagas—even though newborns are tested for 15 diseases that are less common. According to one study, it would be 10 times cheaper to screen and treat babies and their mothers than to cover the costs related to the illness in later years. Few states make the effort.
The gap that enables NTDs to persist, Hernández argues, is the same one that has led to COVID-19 death rates in Black and Latinx communities that are double those elsewhere in America. To close it, she suggests, caring is not enough.
"When I was working on my book," she says, "I thought about HIV in the '80s, when it had so much stigma that no one wanted to talk about it. Then activists stepped up and changed the conversation. I thought a lot about breast cancer, which was stigmatized for years, until people stepped forward and started speaking out. I thought about Lyme disease. And it wasn't only patients—it was also allies, right? The same thing needs to happen with neglected diseases around the world. Allies need to step up and make demands on policymakers. We need to make some noise."
When Wayne Jonas was in medical school 40 years ago, doctors would write out a prescription for placebos, spelling it out backwards in capital letters, O-B-E-C-A-L-P. The pharmacist would fill the prescription with a sugar pill, recalls Jonas, now director of integrative health programs at the Samueli Foundation. It fulfilled the patient's desire for the doctor to do something when perhaps no drug could help, and the sugar pills did no harm.
Today, that deception is seen as unethical. But time and time again, studies have shown that placebos can have real benefits. Now, researchers are trying to untangle the mysteries of placebo effect in an effort to better treat patients.
The use of placebos took off in the post-WWII period, when randomized controlled clinical trials became the gold standard for medical research. One group in a study would be treated with a placebo, a supposedly inert pill or procedure that would not affect normal healing and recovery, while another group in the study would receive an "active" component, most commonly a pill under investigation. Presumably, the group receiving the active treatment would have a better response and the difference from the placebo group would represent the efficacy of the drug being tested. That was the basis for drug approval by the U.S. Food and Drug Administration.
"Placebo responses were marginalized," says Ted Kaptchuk, director of the Program in Placebo Studies & Therapeutic Encounters at Harvard Medical School. "Doctors were taught they have to overcome it when they were thinking about using an effective drug."
But that began to change around the turn of the 21st century. The National Institutes of Health held a series of meetings to set a research agenda and fund studies to answer some basic questions, led by Jonas who was in charge of the office of alternative medicine at the time. "People spontaneously get better all the time," says Kaptchuk. The crucial question was, is the placebo effect real? Is it more than just spontaneous healing?
Brain mechanisms
A turning point came in 2001 in a paper in Science that showed physical evidence of the placebo effect. It used positron emission tomography (PET) scans to measure release patterns of dopamine — a chemical messenger involved in how we feel pleasure — in the brains of patients with Parkinson's disease. Surprisingly, the placebo activated the same patterns that were activated by Parkinson's drugs, such as levodopa. It proved the placebo effect was real; now the search was on to better understand and control it.
A key part of the effect can be the beliefs, expectations, context, and "rituals" of the encounter between doctor and patient. Belief by the doctor and patient that the treatment would work, and the formalized practices of administering the treatment can all contribute to a positive outcome.
Conditioning can be another important component in generating a response, as Pavlov demonstrated more than a century ago in his experiments with dogs. They were trained with a bell prior to feeding such that they would begin to salivate in anticipation at the sound of a bell even with no food present.
Translating that to humans, studies with pain medications and sleeping aids showed that patients who had a positive response with a certain dose of those medications could have the same response if the doses was reduced and a dummy pill substituted, even to the point where there was no longer any active ingredient.
Researchers think placebo treatments can work particularly well in helping people deal with pain and psychological disorders.
Those types of studies troubled Kaptchuk because they often relied on deception; patients weren't told they were receiving a placebo, or at best there was a possibility that they might be randomized to receive a placebo. He believed the placebo effect could work even if patients were told upfront that they were going to receive a placebo. More than a dozen so call "open-label placebo" studies across numerous medical conditions, by Kaptchuk and others, have shown that you don't have to lie to patients for a placebo to work.
Jonas likes to tell the story of a patient who used methotrexate, a potent immunosuppressant, to control her rheumatoid arthritis. She was planning a long trip and didn't want to be bothered with the injections and monitoring required in using the drug, So she began to drink a powerful herbal extract of anise, a licorice flavor that she hated, prior to each injection. She reduced the amount of methotrexate over a period of months and finally stopped, but continued to drink the anise. That process had conditioned her body "to alter her immune function and her autoimmunity" as if she were taking the drug, much like Pavlov's dogs had been trained. She has not taken methotrexate for more than a year.
An intriguing paper published in May found that mild, non-invasive electric stimulation to the brain could not only boost the placebo effect on pain but also reduce the "nocebo" effect — when patients report a negative effect to a sham treatment. While the work is very preliminary, it may open the door to directly manipulating these responses.
Researchers think placebo treatments can work particularly well in helping people deal with pain and psychological disorders, areas where drugs often are of little help. Still, placebos aren't a cure and only a portion of patients experience a placebo effect.
Nocebo
If medicine were a soap opera, the nocebo would be the evil twin of the placebo. It's what happens when patients have adverse side effects because of the expectation that they will. It's commonly seem when patients claims to experience pain or gastric distress that can occur with a drug even when they've received a placebo. The side effects were either imagined or caused by something else.
"Up to 97% of reported pharmaceutical side effects are not caused by the drug itself but rather by nocebo effects and symptom misattribution," according to one 2019 paper.
One way to reduce a nocebo response is to simply not tell patients that specific side effects might occur. An example is a liver biopsy, in which a large-gauge needle is used to extract a tissue sample for examination. Those told ahead of time that they might experience some pain were more likely to report pain and greater pain than those who weren't offered this information.
Interestingly, a nocebo response plays out in the hippocampus, a part of the brain that is never activated in a placebo response. "I think what we are dealing with with nocebo is anxiety," says Kaptchuk, but he acknowledges that others disagree.
Distraction may be another way to minimize the nocebo effect. Pediatricians are using virtual reality (VR) to engage children and distract them during routine procedures such as blood draws and changing wound dressings, and burn patients of all ages have found relief with specially created VRs.
Treatment response
Jonas argues that what we commonly call the placebo effect is misnamed and leading us astray. "The fact is people heal and that inherent healing capacity is both powerful and influenced by mental, social, and contextual factors that are embedded in every medical encounter since the idea of treatment began," he wrote in a 2019 article in the journal Frontiers in Psychiatry. "Our understanding of healing and ability to enhance it will be accelerated if we stop using the term 'placebo response' and call it what it is—the meaning response, and its special application in medicine called the healing response."
He cites evidence that "only 15% to 20% of the healing of an individual or a population comes from health care. The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life."
To better align treatments and maximize their effectiveness, Jonas has created HOPE (Healing Oriented Practices & Environments) Note, "a patient-guided process designed to identify the patient's values and goals in their life and for healing." Essentially, it seeks to make clear to both doctor and patient what the patient's goals are in seeking treatment. In an extreme example of terminal cancer, some patients may choose to extend life despite the often brutal treatments, while others might prefer to optimize quality of life in the remaining time that they have. It builds on practices already taught in medical schools. Jonas believes doctors and patients can use tools like these to maximize the treatment response and achieve better outcomes.
Much of the medical profession has been resistant to these approaches. Part of that is simply tradition and limited data on their effectiveness, but another very real factor is the billing process for how they are reimbursed. Jonas says a new medical billing code added this year gives doctors another way to be compensated for the extra time and effort that a more holistic approach to medicine may initially require. Other moves away from fee-for-service payments to bundling and payment for outcomes, and the integrated care provided by the Veterans Affairs, Kaiser Permanente and other groups offer longer term hope for the future of approaches that might enhance the healing response.

