This Resistance Fighter Invented Dialysis in Nazi-Occupied Holland
When Willem Johan Kolff invented dialysis, the "father" of artificial organs was just getting started.
One of the Netherlands’ most famous pieces of pop culture is “Soldier of Orange.” It’s the title of the country’s most celebrated war memoir, movie and epic stage musical, all of which detail the exploits of the nation’s resistance fighters during World War II.
Willem Johan Kolff was a member of the Dutch resistance, but he doesn’t rate a mention in the “Solider of Orange” canon. Yet his wartime toils in a rural backwater not only changed medicine, but the world.
Kolff had been a physician less than two years before Germany invaded the Netherlands in May 1940. He had been engaged in post-graduate studies at the University of Gronigen but withdrew because he refused to accommodate the demands of the Nazi occupiers. Kolff’s Jewish supervisor made an even starker choice: He committed suicide.
After his departure from the university, Kolff took a job managing a small hospital in Kampen. Located 50 miles from the heavily populated coastal region, the facility was far enough away from the prying eyes of Germans that not only could Kolff care for patients, he could hide fellow resistance fighters and even Jewish refugees in relative safety. Kolff coached many of them to feign convincing terminal illnesses so the Nazis would allow them to remain in the hospital.
Despite the demands of practicing medicine and resistance work, Kolff still found time to conduct research. He had been haunted and inspired when, not long before the Nazi invasion, one of his patients died in agony from kidney disease. Kolff wanted to find a way to save future patients.
He broke his problem down to a simple task: If he could remove 20 grams of urea from a patient’s blood in 24 hours, they would survive. He began experimenting with ways to filter blood and return it to a patient’s body. Since the war had ground all non-military manufacturing to a halt, he was mostly forced to make do with material he could find at the hospital and around Kampen. Kolff eventually built a device from a washing machine parts, juice cans, sausage casings, a valve from an old Ford automobile radiator, and even scrap from a downed German aircraft.
The world’s first dialysis machine was hardly imposing; it resembled a rotating drum for a bingo game or raffle. Yet it carried on the highly sophisticated task of moving a patient’s blood through a semi-permeable membrane (about a 50-foot length of sausage casings) into a saline solution that drew out urea while leaving the blood cells untouched.
In emigrating to the U.S. to practice medicine, Kolff's intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
Kolff began using the machine to treat patients in 1943, most of whom had lapsed into comas due to their kidney failure. But like most groundbreaking medical devices, it was not an immediate success. By the end of the war, Kolff had dialyzed more than a dozen patients, but all had died. He briefly suspended use of the device after the Allied invasion of Europe, but he continued to refine its operation and the administration of blood thinners to patients.
In September 1945, Kolff dialyzed another comatose patient, 67-year-old Sofia Maria Schafstadt. She regained consciousness after 11 hours, and would live well into the 1950s with Kolff’s assistance. Yet this triumph contained a dark irony: At the time of her treatment, Schafstadt had been imprisoned for collaborating with the Germans.
With a tattered Europe struggling to overcome the destruction of the war, Kolff and his family emigrated to the U.S. in 1950, where he began working for the Cleveland Clinic while undergoing the naturalization process so he could practice medicine in the U.S. His intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
By the mid-1950s, dialysis machines had become reliable and life-saving medical devices, and Kolff had become a U.S. citizen. About that time he invented a membrane oxygenator that could be used in heart bypass surgeries. This was a critical component of the heart-lung machine, which would make heart transplants possible and bypass surgeries routine. He also invented among the very first practical artificial hearts, which in 1957 kept a dog alive for 90 minutes.
Kolff moved to the University of Utah in 1967 to become director of its Institute for Biomedical Engineering. It was a promising time for such a move, as the first successful transplant of a donor heart to a human occurred that year. But he was interested in going a step further and creating an artificial heart for human use.
It took more than a decade of tinkering and research, but in 1982, a team of physicians and engineers led by Kolff succeeded in implanting the first artificial heart in dentist Barney Clark, whose failing health disqualified him from a heart transplant. Although Clark died in March 1983 after 112 days tethered to the device, that it kept him alive generated international headlines. While graduate student Robert Jarvik received the named credit for the heart, he was directly supervised by Kolff, whose various endeavors into artificial organ research at the University of Utah were segmented into numerous teams.
Forty years later, several artificial hearts have been approved for use by the Food and Drug Administration, although all are a “bridge” that allow patients to wait for a transplant.
Kolff continued researching and tinkering with biomedical devices – including artificial eyes and ears – until he retired in 1997 at the age of 86. When he died in 2009, the medical community acknowledged that he was not only a pioneer in biotechnology, but the “father” of artificial organs.
New Options Are Emerging in the Search for Better Birth Control
Photo by JPC-PROD on Adobe Stock
About decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April 2021, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
This article was first published by Leaps.org on August 31, 2021.
Hyperbaric oxygen therapy could treat Long COVID, new study shows
Hyperbaric oxygen therapy has been used in the past to help people with traumatic brain injury, stroke and other conditions involving wounds to the brain. Now, researchers at Shamir Medical Center in Tel Aviv are studying how it could treat Long Covid.
Long COVID is not a single disease, it is a syndrome or cluster of symptoms that can arise from exposure to SARS-CoV-2, a virus that affects an unusually large number of different tissue types. That's because the ACE2 receptor it uses to enter cells is common throughout the body, and inflammation from the immune response fighting that infection can damage surrounding tissue.
One of the most widely shared groups of symptoms is fatigue and what has come to be called “brain fog,” a difficulty focusing and an amorphous feeling of slowed mental functioning and capacity. Researchers have tied these COVID-related symptoms to tissue damage in specific sections of the brain and actual shrinkage in its size.
When Shai Efrati, medical director of the Sagol Center for Hyperbaric Medicine and Research in Tel Aviv, first looked at functional magnetic resonance images (fMRIs) of patients with what is now called long COVID, he saw “micro infarcts along the brain.” It reminded him of similar lesions in other conditions he had treated with hyperbaric oxygen therapy (HBOT). “Once we saw that, we said, this is the type of wound we can treat. It doesn't matter if the primary cause is mechanical injury like TBI [traumatic brain injury] or stroke … we know how to oxidize them.”Efrati came to HBOT almost by accident. The physician had seen how it had helped heal diabetic ulcers and improved the lives of other patients, but he was busy with his own research. Then the director of his Tel Aviv hospital threatened to shut down the small HBOT chamber unless Efrati took on administrative responsibility for it. He reluctantly agreed, a decision that shifted the entire focus of his research.
“The main difference between wounds in the leg and wounds in the brain is that one is something we can see, it's tangible, and the wound in the brain is hidden,” says Efrati. With fMRIs, he can measure how a limited supply of oxygen in blood is shuttled around to fuel activity in various parts of the brain. Years of research have mapped how specific areas of the brain control activity ranging from thinking to moving. An fMRI captures the brain area as it’s activated by supplies of oxygen; lack of activity after the same stimuli suggests damage has occurred in that tissue. Suddenly, what was hidden became visible to researchers using fMRI. It helped to make a diagnosis and measure response to treatment.
HBOT is not a single thing but rather a tool, a process or approach with variations depending on the condition being treated. It aims to increase the amount of oxygen that gets to damaged tissue and speed up healing. Regular air is about 21 percent oxygen. But inside the HBOT chamber the atmospheric pressure can be increased to up to three times normal pressure at sea level and the patient breathes pure oxygen through a mask; blood becomes saturated with much higher levels of oxygen. This can defuse through the damaged capillaries of a wound and promote healing.
The trial
Efrati’s clinical trials started in December 2020, barely a year after SARS-CoV-2 had first appeared in Israel. Patients who’d experienced cognitive issues after having COVID received 40 sessions in the chamber over a period of 60 days. In each session, they spent 90 minutes breathing through a mask at two atmospheres of pressure. While inside, they performed mental exercises to train the brain. The only difference between the two groups of patients was that one breathed pure oxygen while the other group breathed normal air. No one knew who was receiving which level of oxygen.
The results were striking. Before and after fMRIs showed significant repair of damaged tissue in the brain and functional cognition tests improved substantially among those who received pure oxygen. Importantly, 80 percent of patients said they felt back to “normal,” but Efrati says they didn't include patient evaluation in the paper because there was no baseline data to show how they functioned before COVID. After the study was completed, the placebo group was offered a new round of treatments using 100 percent oxygen, and the team saw similar results.
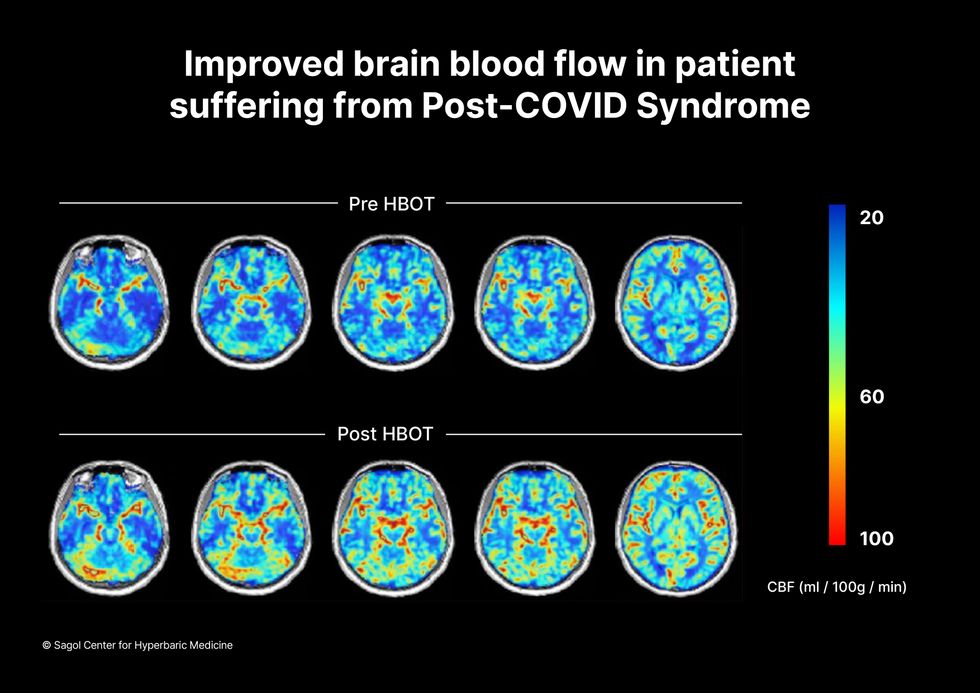
Scans show improved blood flow in a patient suffering from Long Covid.
Sagol Center for Hyperbaric Medicine
Efrati's use of HBOT is part of an emerging geroscience approach to diseases associated with aging. These researchers see systems dysfunctions that are common to several diseases, such as inflammation, which has been shown to play a role in micro infarcts, heart disease and Alzheimer’s disease. Preliminary research suggests that HBOT can retard some underlying mechanisms of aging, which might address several medical conditions. However, the drug approval process is set up to regulate individual disease, not conditions as broad as aging, and so they concentrate on treating the low hanging fruit: disorders where effective treatments currently are limited and success might be demonstrated.
The key to HBOT's effectiveness is something called the hyperoxic-hypoxic paradox where a body does not react to an increase in available oxygen, only to a decrease, regardless of the starting point. That danger signal has a powerful effect on gene expression, resulting in changes in metabolism, and the proliferation of stem cells. That occurs with each cycle of 20 minutes of pure oxygen followed by 5 minutes of regular air circulating through the masks, while the chamber remains pressurized. The high levels of oxygen in the blood provide the fuel necessary for tissue regeneration.
The hyperbaric chamber that Efrati has built can hold a dozen patients and attending medical staff. Think of it as a pressurized airplane cabin, only with much more space than even in first class. In the U.S., people think of HBOT as “a sack of air or some tube that you can buy on Amazon” or find at a health spa. “That is total bullshit,” Efrati says. “It has to be a medical class center where a physician can lose their license if they are not operating it properly.”

Shai Efrati
Alexander Charney, a research psychiatrist at the Icahn School of Medicine at Mount Sinai in New York City, calls Efrati’s study thoughtful and well designed. But it demands a lot from patients with its intense number of sessions. Those types of regimens have proven difficult to roll out to large numbers of patients. Still, the results are intriguing enough to merit additional trials.
John J. Miller, a physician and editor in chief of Psychiatric Times, has seen “many physicians that use hyperbaric oxygen for various brain disorders such as TBI.” He is intrigued by Efrati's work and believes the approach “has great potential to help patients with long COVID whose symptoms are related to brain tissue changes.”
Efrati believes so much in the power of the hyperoxic-hypoxic paradox to heal a variety of tissue injuries that he is leading the medical advisory board at Aviv Clinic, an international network of clinics that are delivering HBOT treatments based on research conducted in Israel. His goal is to silence doubters by quickly opening about 50 such clinics worldwide, based on the model of standalone dialysis clinics in the United States. Sagol Center is treating 300 patients per day, and clinics have opened in Florida and Dubai. There are plans to open another in Manhattan.