Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
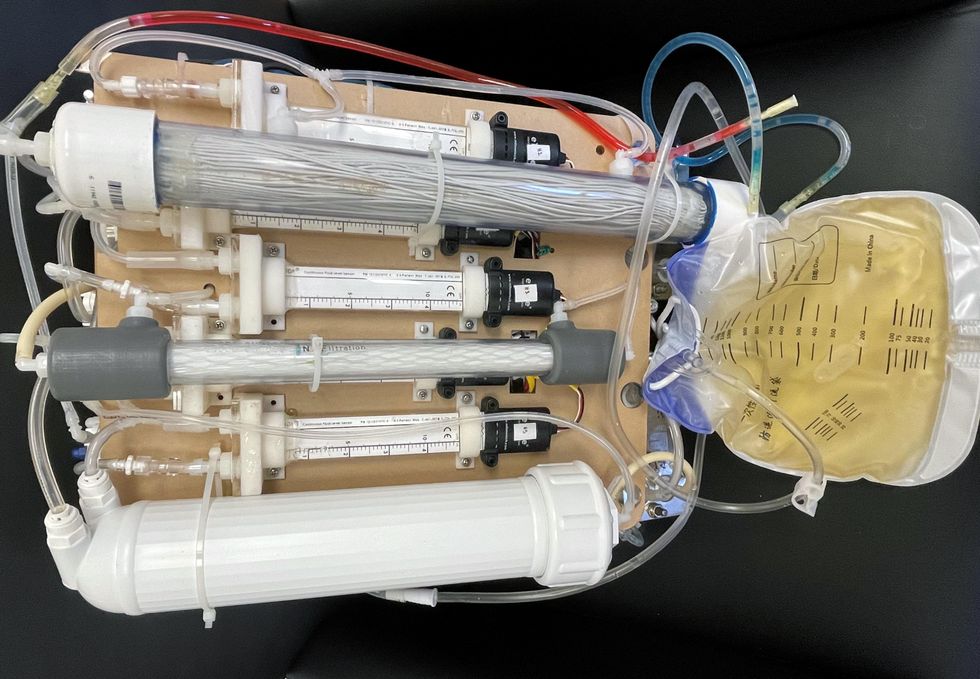
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
Podcast: New Solutions to Combat Gluten Sensitivities and Food Allergies
Biotech company Ukko is designing proteins that will be safe for everyone to eat, starting with peanut and gluten.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
This month, we talk Anat Binur, the CEO of Israeli/U.S.-based biotech company Ukko. Ukko is taking a revolutionary approach to the distressing problem of food allergies and gluten sensitivities: their scientists are designing and engineering proteins that keep the good biophysical properties of the original proteins, while removing the immune-triggering parts that can cause life-threatening allergies. The end goal is proteins that are safe for everyone. Ukko is focusing first on developing a new safe gluten protein for use in baking and a new peanut protein for use as a therapeutic. Their unique platform could theoretically be used for any protein-based allergy, including cats and bees. Hear more in this episode.
Watch the 60-second trailer
Listen to the whole episode
<div id="buzzsprout-player-9950980"></div><script src="https://www.buzzsprout.com/1714953/9950980-solving-food-allergies-with-biotech-company-ukko.js?container_id=buzzsprout-player-9950980&player=small" type="text/javascript" charset="utf-8"></script>
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Can a Non-Invasive Magnetic Helmet Treat Brain Cancer?
Glioblastoma is an aggressive and deadly brain cancer, causing more than 10,000 deaths in the US per year. In the last 30 years there has only been limited improvement in the survival rate despite advances in radiation therapy and chemotherapy. Today the typical survival rate is just 14 months and that extra time is spent suffering from the adverse and often brutal effects of radiation and chemotherapy.
Scientists are trying to design more effective treatments for glioblastoma with fewer side effects, and a team at the Department of Neurosurgery at Houston Methodist Hospital has created a magnetic helmet-based treatment called oncomagnetic therapy: a promising non-invasive treatment for shrinking cancerous tumors. In the first patient tried, the device was able to reduce the tumor of a glioblastoma patient by 31%. The researchers caution, however, that much more research is needed to determine its safety and effectiveness.
How It Works
“The whole idea originally came from a conversation I had with General Norman Schwarzkopf, a supposedly brilliant military strategist,” says David Baskin, professor of neurosurgery and leader of the effort at Houston Methodist. “I asked him what is the secret to your success and he said, ‘Energy. Take out the power grid and the enemy can't communicate.’ So I thought about what supplies [energy to] cancer, especially brain cancer.”
Baskin came up with the idea of targeting the mitochondria, which process and produce energy for cancer cells.
"This is the most exciting thing in glioblastoma treatment I've seen since I've been a neurosurgeon, but it is very preliminary,” Baskin says.
The magnetic helmet creates a powerful oscillating magnetic field. At a set range of frequencies and timings, it disrupts the flow of electrons in the mitochondria of cancer cells. This leads to a release of certain chemicals called Reactive Oxygen Species, or ROS. In normal cells, this excess ROS is much lower, and it's neutralized by other chemicals called antioxidants.
However, cancer cells already have more ROS: they grow rapidly and uncontrollably, so their mitochondria need to produce more energy which in turn generates more ROS. By using the powerful magnetic field, levels of ROS get so high that the malignant cells are torn apart.
The biggest challenge was working out the specific range of frequencies and timing parameters they needed to use to kill cancer cells. It took skill, intuition, luck and lots of experiments. The helmet could theoretically be used to treat all types of glioblastoma.
Developing the magnetic helmet was a collaborative process. Santosh Helekar is a neuroscientist at Houston Methodist Research Institute and the director of oncomagnetics (magnetic cancer therapies) at the Peak Center in Houston Methodist Hospital. His previous invention with colleagues gave the team a starting point to build on. “About 7 years back I developed a portable brain magnetic stimulation device to conduct brain research,” Helekar says. “We [then] conducted a pilot clinical trial in stroke patients. The results were promising.”
Helekar presented his findings to neurosurgeons including Baskin. They decided to collaborate. With a team of scientists behind them, they modified the device to kill cancer cells.

The magnetic helmet studied for treatment of glioblastoma
Dr. David Baskin
Initial Results
After success in the lab, the team got FDA approval to conduct a compassionate trial in a 53-year-old man with end-stage glioblastoma. He had tried every other treatment available. But within 30 days of using the magnetic helmet his tumor shrank by 31%.
Sadly, 36 days into the treatment, the patient had an unrelated head injury due to a fall. The treatment was paused and he later died of the injury. Autopsy results of his brain highlighted the dramatic reduction in tumor cells.
Baskin says, “This is the most exciting thing in glioblastoma treatment I've seen since I've been a neurosurgeon, but it is very preliminary.”
The helmet is part of a growing number of non-invasive cancer treatments. One device that is currently being used by glioblastoma patients is Optune. It uses electric fields called tumor treating fields to slow down cell division and has been through a successful phase 3 clinical trial.
The magnetic helmet has the promise to be another useful non-invasive treatment according to Professor Gabriel Zada, a neurosurgeon and director of the USC Brain Tumor Center. “We're learning that various electromagnetic fields and tumor treating fields appear to play a role in glioblastoma. So there is some precedent for this though the tumor treating fields work a little differently. I think there is major potential for it to be effective but of course it will require some trials.”
Professor Jonathan Sherman, a neurosurgeon and director of neuro-oncology at West Virginia University, reiterates the need for further testing. “It sounds interesting but it’s too early to tell what kind of long-term efficacy you get. We do not have enough data. Also if you’re disrupting [the magnetic field] you could negatively impact a patient. You could be affecting the normal conduction of electromagnetic activity in the brain.”
The team is currently extending their research. They are now testing the treatment in two other patients with end-stage glioblastoma. The immediate challenge is getting FDA approval for those at an earlier stage of the disease who are more likely to benefit.
The Future
Baskin and the team are designing a clinical trial in the U.S., .U.K. and Germany. After positive results in cell cultures, they’re in negotiations to collaborate with other researchers in using the technology for lung and breast cancer. With breast cancer, the soft tissue is easier to access so a magnetic device could be worn over the breast.
“My hope is to develop a treatment to treat and hopefully cure glioblastoma without radiation or chemotherapy,” Baskin says. “We're onto a strategy that could make a huge difference for patients with this disease and probably for patients with many other forms of cancer.”