Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
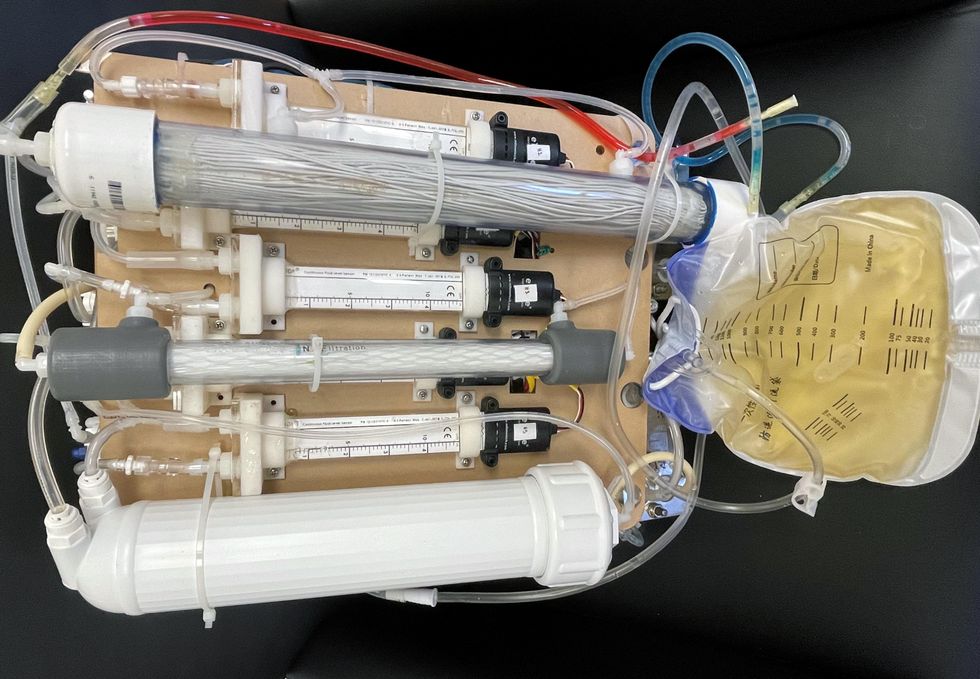
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
Sept. 13th Event: Delta, Vaccines, and Breakthrough Infections
A visualization of the virus that causes COVID-19.
This virtual event will convene leading scientific and medical experts to address the public's questions and concerns about COVID-19 vaccines, Delta, and breakthrough infections. Audience Q&A will follow the panel discussion. Your questions can be submitted in advance at the registration link.
DATE:
Monday, September 13th, 2021
12:30 p.m. - 1:45 p.m. EDT
REGISTER:

Dr. Amesh Adalja, M.D., FIDSA, Senior Scholar, Johns Hopkins Center for Health Security; Adjunct Assistant Professor, Johns Hopkins Bloomberg School of Public Health; Affiliate of the Johns Hopkins Center for Global Health. His work is focused on emerging infectious disease, pandemic preparedness, and biosecurity.

Dr. Nahid Bhadelia, M.D., MALD, Founding Director, Boston University Center for Emerging Infectious Diseases Policy and Research (CEID); Associate Director, National Emerging Infectious Diseases Laboratories (NEIDL), Boston University; Associate Professor, Section of Infectious Diseases, Boston University School of Medicine. She is an internationally recognized leader in highly communicable and emerging infectious diseases (EIDs) with clinical, field, academic, and policy experience in pandemic preparedness.

Dr. Akiko Iwasaki, Ph.D., Waldemar Von Zedtwitz Professor of Immunobiology and Molecular, Cellular and Developmental Biology and Professor of Epidemiology (Microbial Diseases), Yale School of Medicine; Investigator, Howard Hughes Medical Institute. Her laboratory researches how innate recognition of viral infections lead to the generation of adaptive immunity, and how adaptive immunity mediates protection against subsequent viral challenge.
 Dr. Marion Pepper, Ph.D., Associate Professor, Department of Immunology, University of Washington. Her lab studies how cells of the adaptive immune system, called CD4+ T cells and B cells, form immunological memory by visualizing their differentiation, retention, and function.
Dr. Marion Pepper, Ph.D., Associate Professor, Department of Immunology, University of Washington. Her lab studies how cells of the adaptive immune system, called CD4+ T cells and B cells, form immunological memory by visualizing their differentiation, retention, and function.
This event is the third of a four-part series co-hosted by Leaps.org, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Don't Panic Over Waning Antibodies. Here's Why.
A health care worker places a bandaid on the arm of a man who has just been vaccinated.
Since the Delta variant became predominant in the United States on July 7, both scientists and the media alike have been full of mixed messages ("breakthrough infections rare"; "breakthrough infections common"; "vaccines still work"; "vaccines losing their effectiveness") but – if we remember our infectious diseases history- one thing remains clear: immunity is the only way to get through a pandemic.
What Happened in the Past
The 1918 influenza pandemic was far the deadliest respiratory virus pandemic recorded in recent human history with over 50 million deaths (maybe even 100 million deaths, or 3% of the world's population) worldwide. Although they used some of the same measures we are using now (masks, distancing, event closures, as neither testing nor a vaccine existed back then), the deaths slowed only after enough of the population had either acquired immunity through natural infection or died. Indeed, the first influenza vaccine was not developed until 1942, more than 20 years later. As judged by the amount of suffering and death from 1918 influenza (and the deadly Delta surge in India in spring 2021), natural immunity is obviously a terrible way to get through a pandemic.
Similarly, measles was a highly transmissible respiratory virus that led to high levels of immunity among adults who were invariably exposed as children. However, measles led to deaths each year among the nonimmune until a vaccine was developed in 1963, largely restricting current measles outbreaks in the U.S. now to populations who decline to vaccinate. Smallpox also led to high levels of immunity through natural infection, which was often fatal. That's why unleashing smallpox on a largely nonimmune population in the New World was so deadly. Only an effective vaccine – and its administration worldwide, including among populations who declined smallpox vaccine at first via mandates – could control and then eventually eradicate smallpox from Earth.
Fully vaccinated people are already now able to generate some antibodies against all the variants we know of to date, thanks to their bank of memory B cells.
The Delta variant is extremely transmissible, making it unlikely we will ever eliminate or eradicate SARS-CoV-2. Even Australia, which had tried to maintain a COVID-zero nation with masks, distancing, lockdowns, testing and contact tracing before and during the vaccines, ended a strategy aimed at eliminating COVID-19 this week. But, luckily, since highly effective and safe vaccines were developed for COVID-19 less than a year after its advent on a nonimmune population and since vaccines are retaining their effectiveness against severe disease, we have a safe way out of the misery of this pandemic: more and more immunity. "Defanging" SARS-CoV-2 and stripping it of its ability to cause severe disease through immunity will relegate it to the fate of the other four circulating cold-causing coronaviruses, an inconvenience but not a world-stopper.
Immunity Is More Than Antibodies
When we say immunity, we have to be clear that we are talking about cellular immunity and immune memory, not only antibodies. This is a key point. Neutralizing antibodies, which prevent the virus from entering our cells, are generated by the vaccines. But those antibodies will necessarily wane over time since we cannot keep antibodies from every infection and vaccine we have ever seen in the bloodstream (or our blood would be thick as paste!). Vaccines with shorter intervals between doses (like Pfizer vaccines given 3 weeks apart) are likely to have their antibodies wane sooner than vaccines with longer intervals between doses (like Moderna), making mild symptomatic breakthroughs less likely with the Moderna vaccine than the Pfizer during our Delta surge, as a recent Mayo Clinic study showed.
Luckily, the vaccines generate B cells that get relegated to our memory banks and these memory B cells are able to produce high levels of antibodies to fight the virus if they see it again. Amazingly, these memory B cells will actually produce antibodies adapted against the COVID variants if they see a variant in the future, rather than the original antibodies directed against the ancestral strain. This is because memory B cells serve as a blueprint to make antibodies, like the blueprint of a house. If a house needs an extra column (or antibodies need to evolve to work against variants), the blueprint will oblige just as memory B cells will!
One problem with giving a 3rd dose of our current vaccines is that those antibodies won't be adapted towards the variants. Fully vaccinated people are already now able to generate some antibodies against all the variants we know of to date, thanks to their bank of memory B cells. In other words, no variant has evolved to date that completely evades our vaccines. Memory B cells, once generated by either natural infection or vaccination, should be long-lasting.
If memory B cells are formed by a vaccine, they should be as long-lasting as those from natural infection per various human studies. A 2008 Nature study found that survivors of the 1918 influenza pandemic were able to produce antibodies from memory B cells when exposed to the same influenza strain nine decades later. Of note, mild infections (such as the common cold from the cold-causing coronaviruses called 229E, NL63, OC43, and HKU1) may not reliably produce memory B cell immunity like more severe infections caused by SARS-CoV-2.
Right about now, you may be worrying about a super-variant that might yet emerge to evade all our hard-won immune responses. But most immunologists do not think this is very realistic because of T cells. How are T cells different from B Cells? While B cells are like the memory banks to produce antibodies when needed (helped by T cells), T cells will specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly. We likely have T cells to thank for the vaccine's incredible durability in protecting us against severe disease. Data from La Jolla Immunology Institute and UCSF show that the T cell response from the Pfizer vaccine is strong across all the variants.
Think of your spike protein as being comprised of 100 houses with a T cell there to cover each house (to protect you against severe disease). The variants have around 13 mutations along the spike protein so 13 of those T cells won't work, but there are over 80 T cells remaining to protect your "houses" or your body against severe disease.
Although these are theoretical numbers and we don't know exactly the number of T cell antigens (or "epitopes") across the spike protein, one review showed 1400 across the whole virus, with many of those in the spike protein. Another study showed that there were 87 epitopes across the spike protein to which T cells respond, and mutations in one of the variants (beta) took those down to 75. The virus cannot mutate indefinitely in its spike protein and still retain function. This is why it is unlikely we will get a variant that will evade the in-breadth, robust response of our T cells.
Where We Go From Here
So, what does this mean for getting through this pandemic? Immunity and more immunity. For those of us who are vaccinated, if we get exposed to the Delta variant, it will boost our immune response although the memory B cells might take 3-5 days to make new antibodies, which can leave us susceptible to a mild breakthrough infection. That's part of the reason the CDC put back masks for the vaccinated in late July. For those who are unvaccinated, immunity will be gained from Delta but often through terrible ways like severe disease.
The way for the U.S. to determine the need for 3rd shots among those who are not obviously immunocompromised, given the amazing immune memory generated by the vaccines among immunocompetent individuals, is to analyze the cases of the ~6000 individuals who have had severe breakthrough infections among the 171 million Americans fully vaccinated. Define their co-morbidities and age ranges, and boost those susceptible to severe infections (examples could include older people, those with co-morbidities, health care workers, and residents of long-term care facilities). This is an approach likely to be taken by the CDC's Advisory Committee on Immunization Practices.
If immunity is the only way to get through the pandemic and if variants are caused mostly by large populations being unvaccinated, there is not only a moral and ethical imperative but a practical imperative to vaccinate the world in order to keep us all safe. Immunocompetent Americans can boost their antibodies, which may enhance their ability to avoid mild breakthrough infections, but the initial shots still work well against the most important outcomes: hospitalizations and deaths.
There has been no randomized, controlled trial to assess whether three shots vs. two shots meaningfully improve those outcomes. While we ought to trust immune memory to get the immunocompetent in the United States through, we can hasten the end of this pandemic by providing surplus vaccines to poor countries to combat severe disease. Doing so would not only revitalize the role of the U.S. as a global health leader – it would save countless lives.