Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
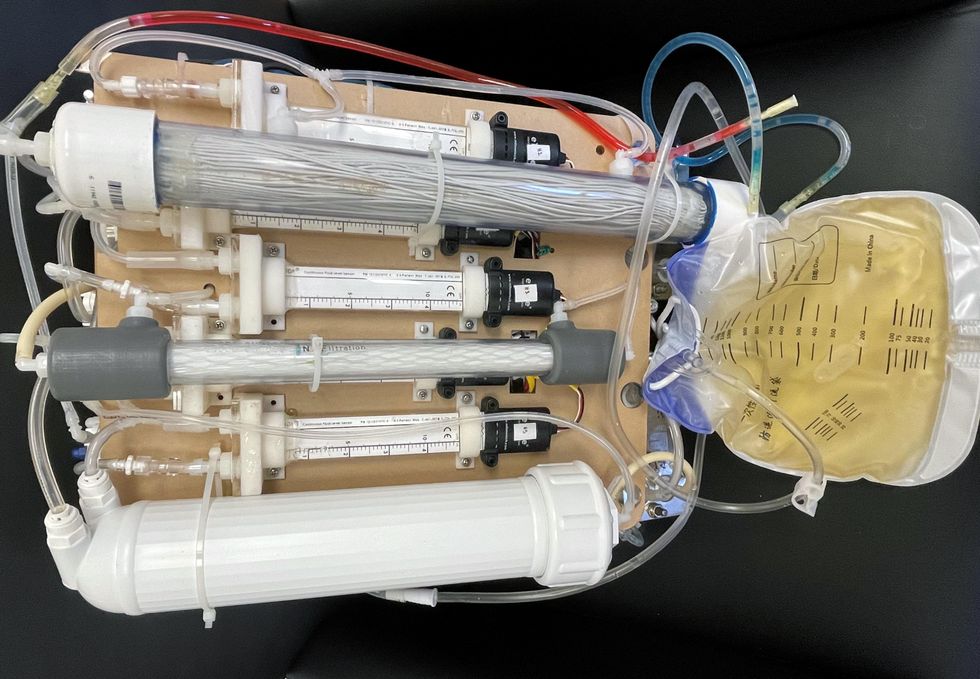
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
An autonomous drone carrying an automated external defibrillator travels to a cardiac emergency in Sweden.
Picture this: your medical first responder descends from the sky like a friendly, unmanned starship. Hovering over your door, it drops a device with recorded instructions to help a bystander jumpstart your heart that has stopped. This, after the 911 call but before the ambulance arrives.
This is exactly what happened on Dec. 9, 2021, when a 71-year-old man in Sweden suffered a cardiac arrest while shoveling snow. A passerby, seeing him collapse, called for an ambulance. In just over three minutes, a drone swooped overhead carrying an Automated External Defibrillator (AED). The patient was revived on the spot before the ambulance arrived to rush him to the hospital where he made a full recovery. The revolutionary technology saved his life.
In 2020, Sweden became the first country to deploy drones carrying AEDs to people in sudden cardiac arrest, when survival odds depend on getting CPR and an electric shock to the heart from a defibrillator within 5 minutes—nearly always before emergency responders arrive.
In the U.S. alone, more than 356,00 cardiac arrests occur outside of hospitals each year; 9 out of 10 of these people die. Plus, the risk of permanent brain injury increases after the first three minutes the heart stops beating. After nine minutes, damage to the brain and other organs is usually severe and irreversible.
“The fundamental technology can be applied to a lot of other emergency situations.”
Once the stuff of sci fi, the delivery of life-saving medical equipment by drone will be commonplace in the near future, experts say. The Swedish team is hailing their study as the first-ever proof of concept for using drones in emergency medicine. The drones arrived only two minutes before the ambulance in most cases but that’s significant during cardiac arrest when survival rates drop 10% every minute.
Since that 2020 pilot, the drones have been tweaked for better performance. They can travel faster and after dark today, and route planning has been optimized, says Mats Sällström, chief executive officer of Everdrone, the technical and development guru for the project, who is collaborating with researchers at the Karolinska Institutet and Sweden’s national emergency call center, SOS Alarm.
When an emergency call comes in, the operator determines if it’s a cardiac arrest. If so, the caller gets CPR instructions while an ambulance is summoned and a control center is notified automatically to dispatch a drone. If conditions allow, the drone flies to the scene via a GPS signal from the caller’s cell phone. Once dropped at the location, the AED beeps to signal its arrival. The AED talks the user through every step when it’s opened while the emergency operator offers support.
Public health officials have tried placing AEDs in public spaces like airports and shopping malls for quick access but the results have been disappointing. Poor usage rates of 2% to 3% have been attributed to bystanders not knowing where they are, not wanting to leave victims, or the site being closed when needed.
Some people fear they could harm the victim or won’t know how to use the AED but not to worry, says Wayne Rosamond, a professor of epidemiology at the University of North Carolina Gillings School of Global Public Health, who studies AED drones. “[The device] won’t shock someone unless they need to be shocked,” he says.
The AED instructions are foolproof, echoes Timothy Chan, professor of engineering at the University of Toronto, who has been building optimization models to design drone networks in Ontario, Canada. All the same, he says, community education will be essential for success. “People have more awareness about drones than AEDs,” he’s found.
Rosamond and Chan are among scientists around the world inspired by Sweden to do their own modeling, simulation and feasibility studies on drone-delivered AEDs.
“Scandinavia is way ahead of us,” notes Rosamond. “There is a tremendous amount of regulatory control over flying drones in the U.S.” In addition to Federal Aviation Administration restrictions, medical drones in the U.S. must comply with HIPAA laws surrounding confidentiality and security of patient information.
To date, Sweden has expanded drone operations and home bases around the country and throughout Europe. Since April 2021, the team has deployed 1-4 drones per week, says Sällström.
Certain weather conditions remain an obstacle. The drones cannot be dispatched safely in rain, snow and heavy wind. Close, heavily populated neighborhoods with high-rise buildings also present challenges.
“Semi-urban areas with residential low-rise [1-5 stories] buildings are the sweet spot for our operations,” Sällström says. “However, as the system matures, we will pursue operations in practically all-weather conditions and also in densely populated areas.” The team is also trying to improve drone speed and battery life to enable flights to rural and remote areas in the future.
Chan predicts that delivering AEDs via drone will be a regular occurrence in five years. In addition, he says, “The fundamental technology can be applied to a lot of other emergency situations.”
Drones could carry medications for anaphylactic shock and opioid overdose, or bring tourniquets and bandages to trauma victims, Chan suggests. Other researchers are looking at the delivery of glucose for low blood sugar emergencies and the transport of organs for transplant.
The sky is no longer the limit.
6 Biotech Breakthroughs of 2021 That Missed the Attention They Deserved
A string of the code that comprises a DNA molecule, pictured at Miraikan, the National Museum of Emerging Science and Innovation in Japan.
News about COVID-19 continues to relentlessly dominate as Omicron surges around the globe. Yet somehow, during the pandemic’s exhausting twists and turns, progress in other areas of health and biotech has marched on.
In some cases, these innovations have occurred despite a broad reallocation of resources to address the COVID crisis. For other breakthroughs, COVID served as the forcing function, pushing scientists and medical providers to rethink key aspects of healthcare, including how cancer, Alzheimer’s and other diseases are studied, diagnosed and treated. Regardless of why they happened, many of these advances didn’t make the headlines of major media outlets, even when they represented turning points in overcoming our toughest health challenges.
If it bleeds, it leads—and many disturbing stories, such as COVID surges, deserve top billing. Too often, though, mainstream media’s parallel strategy seems to be: if it innovates, it fades to the background. But our breakthroughs are just as critical to understanding the state of the world as our setbacks. I asked six pragmatic yet forward-thinking experts on health and biotech for their perspectives on the most important, but under-appreciated, breakthrough of 2021.
Their descriptions, below, were lightly edited by Leaps.org for style and format.
New Alzheimer's Therapies

Mary Carrillo, Chief Science Officer at the Alzheimer’s Association
Alzheimer's Association
One of the biggest health stories of 2021 was the FDA’s accelerated approval of aducanumab, the first drug that treats the underlying biology of Alzheimer’s, not just the symptoms. But, Alzheimer’s is a complex disease and will likely need multiple treatment strategies that target various aspects of the disease. It’s been exciting to see many of these types of therapies advance in 2021.
Following the FDA action in June, we saw renewed excitement in this class of disease-modifying drugs that target beta-amyloid, a protein that accumulates in the brain and leads to brain cell death. This class includes drugs from Eli Lilly (donanemab), Eisai (lecanemab) and Roche (gantenerumab), all of which received Breakthrough Designation by the FDA in 2021, advancing the drugs more quickly through the approval process.
We’ve also seen treatments advance that target other hallmarks of Alzheimer’s this year. We heard topline results from a phase 2 trial of semorinemab, a drug that targets tau tangles, a toxic protein that destroys neurons in the Alzheimer’s brain. Plus, strategies targeting neuroinflammation, protecting brain cells, and reducing vascular contributions to dementia – all funded through the Alzheimer's Association Part the Cloud program – advanced into clinical trials.
The future of Alzheimer’s treatment will likely be combination therapy, including drug therapies and healthy lifestyle changes, similar to how we treat heart disease. Washington University announced they will be testing a combination of both anti-amyloid and anti-tau drugs in a first-of-its-kind clinical trial, with funding from the Alzheimer’s Association.
AlphaFold

Olivier Elemento, Director of the Caryl and Israel Englander Institute for Precision Medicine at Cornell University
Cornell University
AlphaFold is an artificial intelligence system designed by Google’s DeepMind that opens the door to understanding the three-dimensional structures and functions of proteins, the building blocks that make up almost half of our bodies' dry weight. In 2021, Google made AlphaFold available for free and since then, researchers have used it to drive greater understanding of how proteins interact. This is a foundational event in the field of biotech.
It’s going to take time for the benefits from AlphaFold to transpire, but once we know the 3-D structures of proteins that cause various diseases, it will be much easier to design new drugs that can bind to these proteins and change their activity. Prior to AlphaFold, scientists had identified the 3-D structure of just 17 percent of about 20,000 proteins in the body, partly because mapping the structures was extremely difficult and expensive. Thanks to AlphaFold, we’ve now jumped to knowing – with at least some degree of certainty – the protein structures of 98.5 percent of the proteome.
For example, kinases are a class of proteins that modify other proteins and are often aberrantly active in cancer due to DNA mutations. Some of the earliest targeted therapies for cancer were ones that block kinases but, before AlphaFold, we had only a premature understanding of a few hundred kinases. We can now determine the structures of all 1,500 kinases. This opens up a universe of drug targets we didn’t have before.
Additional progress has been made this year toward potentially using AlphaFold to develop blockers of certain protein receptors that contribute to psychiatric illnesses and other neurological diseases. And in July, scientists used AlphaFold to map the dimensions of a bacterial protein that may be key to countering antibiotic resistance. Another discovery in May could be essential to finding treatments for COVID-19. Ongoing research is using AlphaFold principles to create entirely new proteins from scratch that could have therapeutic uses. The AlphaFold revolution is just beginning.
Virtual First Care

Jennifer Goldsack, CEO of Digital Medicine Society
Digital Medicine Society
Imagine a new paradigm of healthcare defined by how good we are at keeping people healthy and out of the clinic, not how good we are at offering services to a sick person at the clinic. That is the promise of virtual-first care, or V1C, what I consider to be the greatest, and most underappreciated, advance that occurred in medicine this year.
V1C is defined as medical care accessed through digital interactions where possible, guided by a clinician, and integrated into a person’s everyday life. This type of care includes spit kits mailed for laboratory tests and replacing in-person exams with biometric sensors. It’s built around the patient, not the clinic, and provides us with the opportunity to fundamentally reimagine what good healthcare looks like.
V1C flew under the radar in 2021, eclipsed by the ongoing debate about the value of telehealth more broadly as we emerge from the pandemic. However, the growth in the number of specialty and primary care virtual-first providers has been matched only by the number of national health plans offering virtual-first plans. Our own virtual-first community, IMPACT, has tripled in size, mirroring the rapid growth of the field driven by patient demand for care on their terms.
V1C differs from the ‘bolt on’ approach of video visits as an add-on to traditional visit-based, episodic care. V1C takes a much more holistic approach; it allows individuals to initiate care at any time in any place, recognizing that healthcare needs extend beyond 9-5. It matches the care setting with each individual’s clinical needs and personal preferences, advancing a thorough, evidence-based, safe practice while protecting privacy and recognizing that patients’ expectations have changed following the pandemic. V1C puts the promise of digital health into practice. This is the blueprint for what good healthcare looks like in the digital era.
Digital Clinical Trials

Craig Lipset, Founder of Clinical Innovation Partners and former Head of Clinical Innovation at Pfizer
Craig Lipset
In 2021, a number of digital- and data-enabled approaches have sustained decentralized clinical trials around the world for many different disease types. Pharma companies and clinical researchers are enthusiastic about this development for good reason. Throughout the pandemic, these decentralized trials have allowed patients to continue in studies with a reduced need for site visits, without compromising their safety or data quality.
Risk-based monitoring was deployed using data and thoughtful algorithms to identify quality and safety issues without relying entirely on human monitors visiting research sites. Some trials used digital measures to ensure high quality data on target health outcomes that could be captured in ways that made the participants’ physical location irrelevant. More than three-quarters of research organizations, such as pharma and biotech, have accelerated their decentralized clinical trial strategies. Before COVID-19, 72 percent of trial sites “rarely or never” used telemedicine for trial participants; during COVID, 64 percent “sometimes, often or always” do.
While the research community does appreciate the tremendous hope and promise brought by these innovations, perhaps what has been under-appreciated is the culture shift toward thoughtful risk-taking and a willingness to embrace and adopt clinical trial innovations. These solutions existed before COVID, but the pandemic shifted the perception of risks versus benefits involved in these trials. If there is one breakthrough that is perhaps under-appreciated in life sciences clinical research today, it’s the power of this new culture of willingness and receptivity to outlast the pandemic. Perhaps the greatest loss to the research ecosystem would be if we lose the momentum with recent trial innovations and must wait for another global pandemic in order to see it again.
Designing Biology

Sudip Parikh, CEO of the American Association for the Advancement of Science and Executive Publisher of the Science family of journals
American Association for the Advancement of Science
As our understanding of basic biology has grown, we are fast approaching an era where it will be possible to design and direct biological machinery to create treatments, medicine, and materials. 2021 saw many breakthroughs in this area, three of which are listed below.
The understanding of the human microbiome is growing as is our ability to modify it. One example is the movement toward the notion of the “bug as the drug.” In June, scientists at the Brigham and Women’s Hospital published a paper showing that they had genetically engineered yeast – using CRISPR/Cas9 – to sense and treat inflammation in the body to relieve symptoms of irritable bowel syndrome in mice. This approach could potentially be used to address issues with your microbiome to treat other chronic conditions.
Another way in which we saw the application of basic biology discoveries to real world problems in 2021 is through groundbreaking research on synthetic biology. Several institutions and companies are pursuing this path. Ginkgo Bioworks, valued at $15 billion, already claims to engineer cells with assembly-line efficiency. Imagine the possibilities of programming cells and tissue to perform chemistry for the manufacturing process, inspired by the way your body does chemistry. That could mean cleaner, more controllable, and affordable ways to manufacture food, therapeutics, and other materials in a factory-like setting.
A final example: consider the possibility of leveraging the mechanics of your own body to deliver proteins as treatments, vaccines, and more. In 2021, several scientists accelerated research to apply the mRNA technology underlying COVID-19 vaccines to make and replace proteins that, when they’re missing or don’t work, cause rare conditions such as cystic fibrosis and multiple sclerosis.
These applications of basic biology to solve real world problems are exciting on their own, but their convergence with incredible advances in computing, materials, and drug delivery hold the promise of game-changing progress in health care and beyond.
Brain Biomarkers

David R. Walt, Professor of Biologically Inspired Engineering, Harvard Medical School, Brigham and Women’s Hospital, Wyss Institute at Harvard University
David Walt
2021 brought the first real hope for identifying biomarkers that can predict neurodegenerative disease. Multiple biomarkers (which are measurable indicators of the presence or severity of disease) were identified that can diagnose disease and that correlate with disease progression. Some of these biomarkers were detected in cerebrospinal fluid (CSF) but others were measured directly in blood by examining precursors of protein fibers.
The blood-brain barrier prevents many biomolecules from both exiting and entering the brain, so it has been a longstanding challenge to detect and identify biomarkers that signal changes in brain chemistry due to neurodegenerative disease. With the advent of omics-based approaches (an emerging field that encompasses genomics, epigenomics, transcriptomics, proteomics, and metabolomics), coupled with new ultrasensitive analytical methods, researchers are beginning to identify informative brain biomarkers. Such biomarkers portend our ability to detect earlier stages of disease when therapeutic intervention could be effective at halting progression.
In addition, these biomarkers should enable drug developers to monitor the efficacy of candidate drugs in the blood of participants enrolled in clinical trials aimed at slowing neurodegeneration. These biomarkers begin to move us away from relying on cognitive performance indicators and imaging—methods that do not directly measure the underlying biology of neurodegenerative disease. The identity of these biomarkers may also provide researchers with clues about the causes of neurodegenerative disease, which can serve as new targets for drug intervention.

