Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
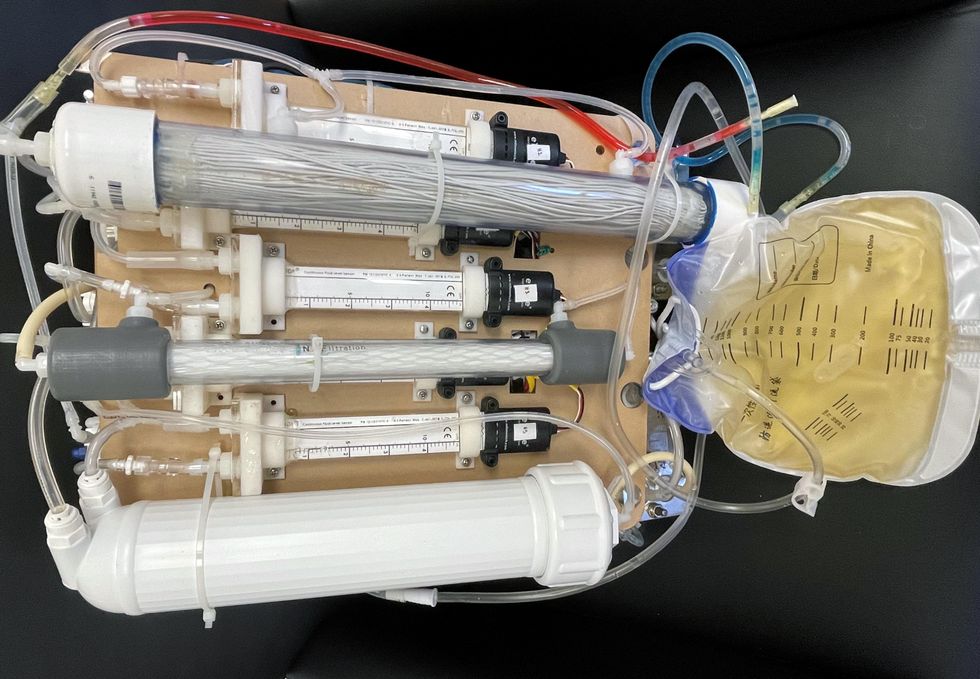
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
Naked Mole Rats Defy Aging. One Scientist Has Dedicated Her Career to Finding Out How.
Naked mole rats have extraordinarily long lifespans and are extremely resistant to cancer.
Rochelle "Shelley" Buffenstein has one of the world's largest, if not the largest, lab-dwelling colonies of the naked mole rat. (No one has done a worldwide tabulation, but she has 4,500 of them.) Buffenstein has spent decades studying the little subterranean-dwelling rodents. Over the years, she and her colleagues have uncovered one surprising discovery after another, which has led them to re-orient the whole field of anti-aging research.
Naked mole rats defy everything we thought we knew about aging. These strange little rodents from arid regions of Africa, such as Kenya, Ethiopia and Somalia, live up to ten times longer than their size would suggest. And unlike virtually every other animal, they don't lose physical or cognitive abilities with age, and even retain their fertility up until the end of life. They appear to have active defenses against the ravages of time, suggesting that aging may not be inevitable. Could these unusual creatures teach humans how to extend life and ameliorate aging?
Buffenstein, who is senior principle investigator at Calico Life Sciences, has dedicated her life to finding out. Her early interest in the animals of what is now Zimbabwe led to her current position as a cutting-edge anti-aging researcher at Calico, the Google-funded health venture launched in 2013. The notoriously secretive company is focused on untangling the mysteries of why animals and people age, and whether there are ways to slow or temporarily arrest the process.
The small, wrinkly animal, which lives in underground burrows in the hot, arid regions of Africa, is hardly the beauty queen of the mammalian kingdom. Furless, buck-toothed and tiny-eyed, the creatures look like they could use a good orthodontist, a protective suit of clothes and possibly, some spectacles to enhance their eyesight. But these rats more than make up for their unimpressive looks with their superlative ability to adapt to some of the most inhospitable conditions on earth.
Based on the usual rule that body size predicts lifespan, naked mole rats shouldn't live that long. After all, similarly-sized rodents like mice have a life expectancy of two years or less. But Buffenstein was one of the first scientists to recognize that naked mole rats live an extraordinarily long time, with her oldest animal approaching 39 years of age. In addition, they never become geriatric in the human sense, defying the common signs of aging — age-related diseases, cognitive decline and even menopause. In fact, the queens, or females that do all the breeding in a bee-like underground colony, remain fertile and give birth to healthy pups up until what would be considered very old age in humans. And the naked mole rat has other curious abilities, such as the ability to endure extreme low-oxygen, or hypoxic, conditions like those they encounter in their underground nests.
"One thing we've learned from these animals is that they stay healthy until the very end."
It's not that the naked mole rat isn't subject to the vicissitudes of life, or the normal wear and tear of biological processes. Over the years, Buffenstein and her colleagues have discovered that, while the process of oxidative stress — thought for 50 years to be the main cause of aging — occurs in the naked mole rat just as in any other animal, its damage does not accumulate with age. Oxidative stress occurs during normal cell metabolism when oxygen "free radicals" with one or more unpaired electrons wreak havoc on large cellular molecules, leaving microscopic debris in their wake that clogs up the gears of healthy cell function. Somehow, naked mole rats have an enhanced ability to clear out the damaged cells and molecules before they can set off the usual chain reaction of cell dysfunction and death, according to a 2013 paper in which Buffenstein is the lead author.
Oxidative stress is not the only factor known to be problematic in aging. Slowly accumulating damage to DNA typically leads to protein malfunction and improper folding. In humans and most other animals, these protein fragments can accumulate in cells and gum up the works. Only not so much in naked mole rats, which are able to maintain normal protein folding throughout their long life. After years of discoveries like these, Buffenstein has gradually reframed her focus from "what goes wrong to produce aging?" to "what goes right in the naked mole rat to help it defy the normal wear and tear of life?" Buffenstein's research suggests that the tiny mammals have a unique ability to somehow clear out damaged protein fragments and other toxic debris before they can cause disease and aging.
How She Got Here
Buffenstein ascribes her initial acquaintance with the naked mole rat to serendipity. Back in 1979, her postgraduate mentor Jenny Jarvis at the University of Cape Town in South Africa kept a small colony of rats in her office while studying the mechanisms that lead to the animals' unusual adaptive capabilities. It was Buffenstein's job to take care of them. Working with Jarvis, Buffenstein focused on understanding their unique adaptations to the extreme conditions of their natural habitat.
They studied the unusual behaviors regulating the rat colonies. For instance, they observed that designated "workers" dig the entire colony's underground tunnels and a single reproducing female breeds with only a small number of males. Buffenstein also examined how these animals are able to survive without the "sunshine hormone" — vitamin D — and their unusual modes of regulating their internal temperatures and converting food into energy. Though classified as mammals, the rodents simply don't conform to the mammalian handbook, having found ingenuous ways to alter their bodies and behavior that is fine-tuned to the scorching heat and aridity of their environment.
To escape the heat, they simply burrow underground and live in elaborate tunnels. To cope with the low-oxygen conditions underground, they slowed their metabolism and learned to live for extended periods of time in such hypoxic conditions that an ordinary animal would quickly suffocate. But it was slowly dawning on Buffenstein that the small creatures were exceptional in additional ways.
When Buffenstein got her first academic position at the University of Witwatersrand in Johannesburg, Jarvis said she could take some of the naked mole rats with her. When she did, Buffenstein noticed that the animals were living far longer than similarly sized rodents. "At that stage, they were about ten years old. Little did I know how long they would eventually show us they could live," she says.
In 1997, after accepting a position at the City College of New York, Buffenstein moved to the U.S. and took her rat colony with her. There she was able to pursue an evolving narrative about the humble naked mole rat that continued to defy expectations. As the years passed, it was becoming more and more evident that her observations could have major implications for aging research. Eventually, she took a position at the Barshop Institute for Aging and Longevity Studies in San Antonio, Texas.
One early observation of Buffenstein's suggested that the species most often used in aging research—mice, roundworms, fruit flies and yeast—have short lifespans and poor defenses against aging. These animals provide important insights into how aging works, and have revealed possible targets for intervention. But they don't show what goes right in apparently non-aging animals like the naked mole rat.
Buffenstein's years of studying the rats has laid the foundation for a whole new perspective in aging research.
"My hypothesis," she says, "is that naked mole rats are very good at removing damaged macromolecules and cells, thereby maintaining homeostasis and cell and tissue function. All the repair pathways examined by us and others in the field point to more efficient repair and more rapid responses to damaging agents." These include things like free radicals and radiation.
Buffenstein’s Legacy
Some researchers today are building on Buffenstein's foundational discoveries to home in on possible anti-aging mechanisms that lead to the extraordinary resilience of naked mole rats. University of Cambridge researcher and co-founder of the institution's Naked Mole-Rat Initiative, Ewan St. John Smith, is studying the animal's resistance to cancer.
In a 2020 paper published in Nature, Smith and his colleagues established that naked mole rats harbor cancer-causing genes, and these genes occasionally create cancer cells. But something in the rats shuts the multiplication process down before the cells can grow out of control and form tumors. Now, scientists want to know what mechanisms, exactly, are at play in preventing the cells from invading healthy tissues. Smith has hypothesized that the answer is somehow embedded in interactions in the cells' microenvironment.
He also thinks the animal's immune system could just be very effective at seeking out and destroying cancer cells. Several current cancer therapies work by boosting the body's immune system so it can attack and eliminate the toxic cells. It's possible that the naked mole rat's immune system naturally goes into hyper-drive when cancer cells appear, enabling it to nip the disease in the bud before tumors can form. A pharmacologist by training, Smith thinks that if there is some chemical mediator in the naked mole rat that supercharges its immune cells, perhaps that mediator can be synthesized in a drug to treat humans for cancer.
The naked mole rat's extreme tolerance to hypoxia could also play a role. "Interestingly," he says, "when cells become cancerous, they also become hypoxic, and naked mole rats are known to be very resistant to hypoxia.
He notes that a form of low-level hypoxia is also present in the bodies and brains of both aged mice and older humans. It's commonly seen in the brains of humans with Alzheimer's disease and other forms of age-related dementia. This suggests that hypoxia in humans — and in other mammals — may have a role to play in Alzheimer's and the aging process itself. Resistance to hypoxia could be why the naked mole rat, in Smith's words, "chugs along quite happily" in conditions that in humans are associated with disease and decline.
Smith cheerfully acknowledges his debt to Buffenstein for laying so much of the groundwork in a field rife with possible implications for anti-aging. "Shelley is amazing," he says. "Naked mole rats have a queen and I always refer to her as the queen of the naked mole rat world." In fact, Buffenstein gave Smith his first colony of rats, which he's since grown to about 150. "Some of them will still be around when I retire," he jokes.
Vera Gorbunova, a professor of biology and oncology at the University of Rochester who studies both longevity and cancer in naked mole rats, credits Buffenstein with getting others to study the animals for anti-aging purposes. Gorbunova believes that "cancer and aging go hand-in-hand" and that longer-lived animals have better, more accurate DNA repair.
Gorbunova is especially interested in the naked mole rat's ability to secrete a superabundance of a "super-sugar" molecule called hyaluronan, a ubiquitous additive to skin creams for its moisturizing effect. Gorbunova and others have observed that the presence of high concentrations of hyaluronan in the naked mole rat's extracellular matrix — the chemical-rich solution between cells — prevents the overcrowding of cells. This, perhaps, could be the key to the animal's ability to stop tumors from forming.
Hyaluronan is also present in the extracellular matrix of humans, but the naked mole rat molecule is more than five times larger than the versions found in humans or mice, and is thought to play a significant part in the animal's DNA repair. But just rubbing a cream containing hyaluronan over your skin won't stop cancer or aging. High concentrations of the substance in the extracellular matrix throughout your body would likely be needed.
Gorbunova notes that the naked mole rat offers a multitude of possibilities that could eventually lead to drugs to slow human aging. "I'm optimistic that there are many different strategies, because the naked mole rat likely has many processes going on that fight aging," she says. "I think that in a relatively short time, there will be bonafide treatments to test in animals. One thing we've learned from these animals is that they stay healthy until the very end."
So if naked mole rats don't become frail with age or develop age-related diseases, what does kill them? The answer, unfortunately, is usually other naked mole rats. Buffenstein has long noted that even though they live in highly cooperative colonies, they can be quite cantankerous when there's a disruption in the hierarchy, a sentiment echoed by Gorbunova. "Sometimes there are periods of peace and quiet, but if something happens to the queen, all hell breaks loose," she says. "If the queen is strong, everybody knows their place," but if the queen dies, the new queen is inevitably decided by violent competition.
To the casual observer, a strange, wrinkly rodent like the naked mole rat might seem to have little to teach us about ourselves, but Buffenstein is confident that her discoveries could have major implications for human longevity research. Today, at Calico's labs in San Francisco, she's focused entirely on the determining how anti-aging defense mechanisms in the rats could lead to similar defenses being stimulated or introduced in humans.
"The million-dollar question is, what are the mechanisms protecting against aging, and can these be translated into therapies to delay or abrogate human aging, too?"
Buffenstein fired up a new generation of scientists with multiple discoveries, especially the fundamental one that naked mole rats are subject to the same wear and tear over time as the rest of us, but somehow manage to reverse it. These days, the trailblazer is at work on untangling the molecular mechanisms involved in the animal's resistance to cardiac aging. On top of everything else, the small creature has a unique ability to fight off the scourge of heart disease, which is the leading cause of death in the industrialized world.
After all, the point is not to extend old age, but to slow down aging itself so that frailty and disability are compressed into a brief period after a long-extended period of vitality. By switching the focus from what goes wrong to mechanisms that defend against aging in the first place, the discoveries of Buffenstein and a new generation of researchers who are building on her groundbreaking research promise to be a driving force in the quest to extend not only life, but healthy, vigorous life in humans.
This article was first published by Leaps.org on June 23, 2021.
How mRNA Could Revolutionize Medicine
In November 2020, messenger RNA catapulted into the public consciousness when the first COVID-19 vaccines were authorized for emergency use. Around the same time, an equally groundbreaking yet relatively unheralded application of mRNA technology was taking place at a London hospital.
Over the past two decades, there's been increasing interest in harnessing mRNA — molecules present in all of our cells that act like digital tape recorders, copying instructions from DNA in the cell nucleus and carrying them to the protein-making structures — to create a whole new class of therapeutics.
Scientists realized that artificial mRNA, designed in the lab, could be used to instruct our cells to produce certain antibodies, turning our bodies into vaccine-making factories, or to recognize and attack tumors. More recently, researchers recognized that mRNA could also be used to make another groundbreaking technology far more accessible to more patients: gene editing. The gene-editing tool CRISPR has generated plenty of hype for its potential to cure inherited diseases. But delivering CRISPR to the body is complicated and costly.
"Most gene editing involves taking cells out of the patient, treating them and then giving them back, which is an extremely expensive process," explains Drew Weissman, professor of medicine at the University of Pennsylvania, who was involved in developing the mRNA technology behind the COVID-19 vaccines.
But last November, a Massachusetts-based biotech company called Intellia Therapeutics showed it was possible to use mRNA to make the CRISPR system inside the body, eliminating the need to extract cells out of the body and edit them in a lab. Just as mRNA can instruct our cells to produce antibodies against a viral infection, it can also teach them to produce one of the two components that make up CRISPR — a cutting protein that snips out a problem gene.
"The pandemic has really shown that not only are mRNA approaches viable, they could in certain circumstances be vastly superior to more traditional technologies."
In Intellia's London-based clinical trial, the company applied this for the first time in a patient with a rare inherited liver disease known as hereditary transthyretin amyloidosis with polyneuropathy. The disease causes a toxic protein to build up in a person's organs and is typically fatal. In a company press release, Intellia's president and CEO John Leonard swiftly declared that its mRNA-based CRISPR therapy could usher in a "new era of potential genome editing cures."
Weissman predicts that turning CRISPR into an affordable therapy will become the next major frontier for mRNA over the coming decade. His lab is currently working on an mRNA-based CRISPR treatment for sickle cell disease. More than 300,000 babies are born with sickle cell every year, mainly in lower income nations.
"There is a FDA-approved cure, but it involves taking the bone marrow out of the person, and then giving it back which is prohibitively expensive," he says. It also requires a patient to have a matched bone marrow done. "We give an intravenous injection of mRNA lipid nanoparticles that target CRISPR to the bone marrow stem cells in the patient, which is easy, and much less expensive."
Cancer Immunotherapy
Meanwhile, the overwhelming success of the COVID-19 vaccines has focused attention on other ways of using mRNA to bolster the immune system against threats ranging from other infectious diseases to cancer.
The practicality of mRNA vaccines – relatively small quantities are required to induce an antibody response – coupled with their adaptable design, mean companies like Moderna are now targeting pathogens like Zika, chikungunya and cytomegalovirus, or CMV, which previously considered commercially unviable for vaccine developers. This is because outbreaks have been relatively sporadic, and these viruses mainly affect people in low-income nations who can't afford to pay premium prices for a vaccine. But mRNA technology means that jabs could be produced on a flexible basis, when required, at relatively low cost.
Other scientists suggest that mRNA could even provide a means of developing a universal influenza vaccine, a goal that's long been the Holy Grail for vaccinologists around the world.
"The mRNA technology allows you to pick out bits of the virus that you want to induce immunity to," says Michael Mulqueen, vice president of business development at eTheRNA, a Belgium-based biotech that's developing mRNA-based vaccines for malaria and HIV, as well as various forms of cancer. "This means you can get the immune system primed to the bits of the virus that don't vary so much between strains. So you could actually have a single vaccine that protects against a whole raft of different variants of the same virus, offering more universal coverage."
Before mRNA became synonymous with vaccines, its biggest potential was for cancer treatments. BioNTech, the German biotech company that collaborated with Pfizer to develop the first authorized COVID-19 vaccine, was initially founded to utilize mRNA for personalized cancer treatments, and the company remains interested in cancers ranging from melanoma to breast cancer.
One of the major hurdles in treating cancer has been the fact that tumors can look very different from one person to the next. It's why conventional approaches, such as chemotherapy or radiation, don't work for every patient. But weaponizing mRNA against cancer primes the immune cells with the tumor's specific genetic sequence, training the patient's body to attack their own unique type of cancer.
"It means you're able to think about personalizing cancer treatments down to specific subgroups of patients," says Mulqueen. "For example, eTheRNA are developing a renal cell carcinoma treatment which will be targeted at around 20% of these patients, who have specific tumor types. We're hoping to take that to human trials next year, but the challenge is trying to identify the right patients for the treatment at an early stage."
Repairing Damaged mRNA
While hopes are high that mRNA could usher in new cancer treatments and make CRISPR more accessible, a growing number of companies are also exploring an alternative to gene editing, known as RNA editing.
In genetic disorders, the mRNA in certain cells is impaired due to a rogue gene defect, and so the body ceases to produce a particular vital protein. Instead of permanently deleting the problem gene with CRISPR, the idea behind RNA editing is to inject small pieces of synthetic mRNA to repair the existing mRNA. Scientists think this approach will allow normal protein production to resume.
Over the past few years, this approach has gathered momentum, as some researchers have recognized that it holds certain key advantages over CRISPR. Companies from Belgium to Japan are now looking at RNA editing to treat all kinds of disorders, from Huntingdon's disease, to amyotrophic lateral sclerosis, or ALS, and certain types of cancer.
"With RNA editing, you don't need to make any changes to the DNA," explains Daniel de Boer, CEO of Dutch biotech ProQR, which is looking to treat rare genetic disorders that cause blindness. "Changes to the DNA are permanent, so if something goes wrong, that may not be desirable. With RNA editing, it's a temporary change, so we dose patients with our drugs once or twice a year."
Last month, ProQR reported a landmark case study, in which a patient with a rare form of blindness called Leber congenital amaurosis, which affects the retina at the back of the eye, recovered vision after three months of treatment.
"We have seen that this RNA therapy restores vision in people that were completely blind for a year or so," says de Boer. "They were able to see again, to read again. We think there are a large number of other genetic diseases we could go after with this technology. There are thousands of different mutations that can lead to blindness, and we think this technology can target approximately 25% of them."
Ultimately, there's likely to be a role for both RNA editing and CRISPR, depending on the disease. "I think CRISPR is ideally suited for illnesses where you would like to permanently correct a genetic defect," says Joshua Rosenthal of the Marine Biology Laboratory in Chicago. "Whereas RNA editing could be used to treat things like pain, where you might want to reset a neural circuit temporarily over a shorter period of time."
Much of this research has been accelerated by the COVID-19 pandemic, which has played a major role in bringing mRNA to the forefront of people's minds as a therapeutic.
"The pandemic has really shown that not only are mRNA approaches viable, they could in certain circumstances be vastly superior to more traditional technologies," says Mulqueen. "In the future, I would not be surprised if many of the top pharma products are mRNA derived."

