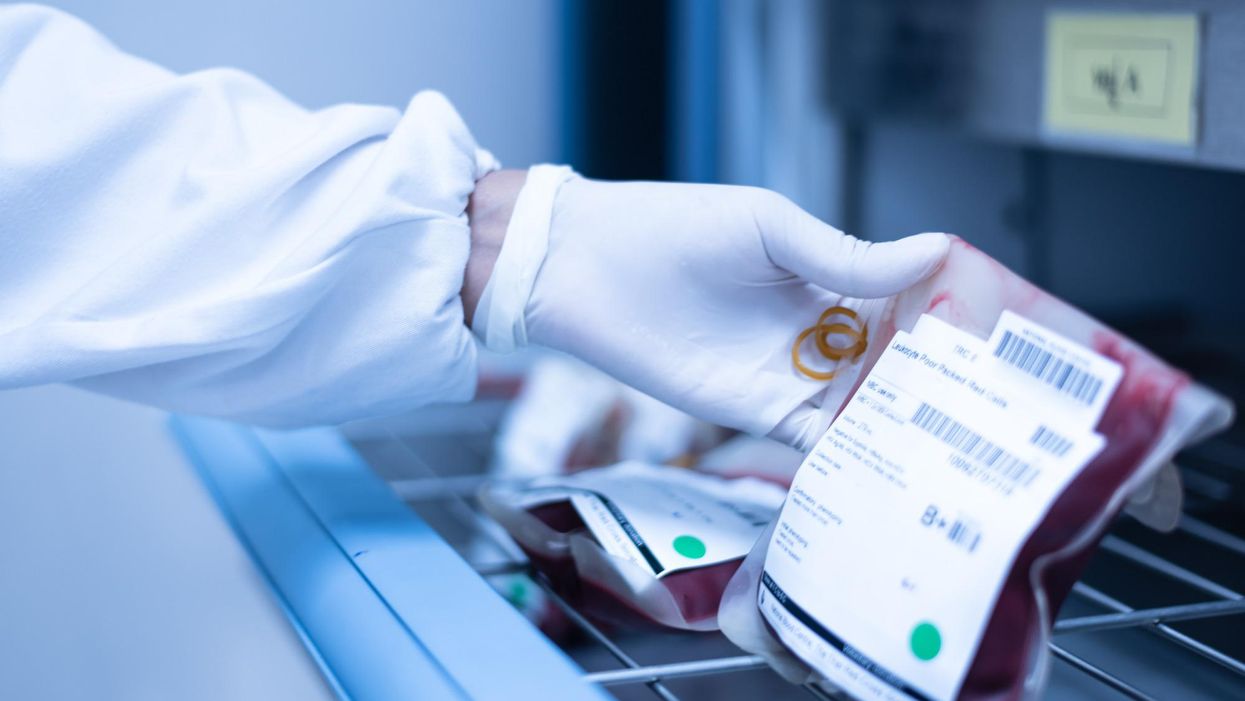
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
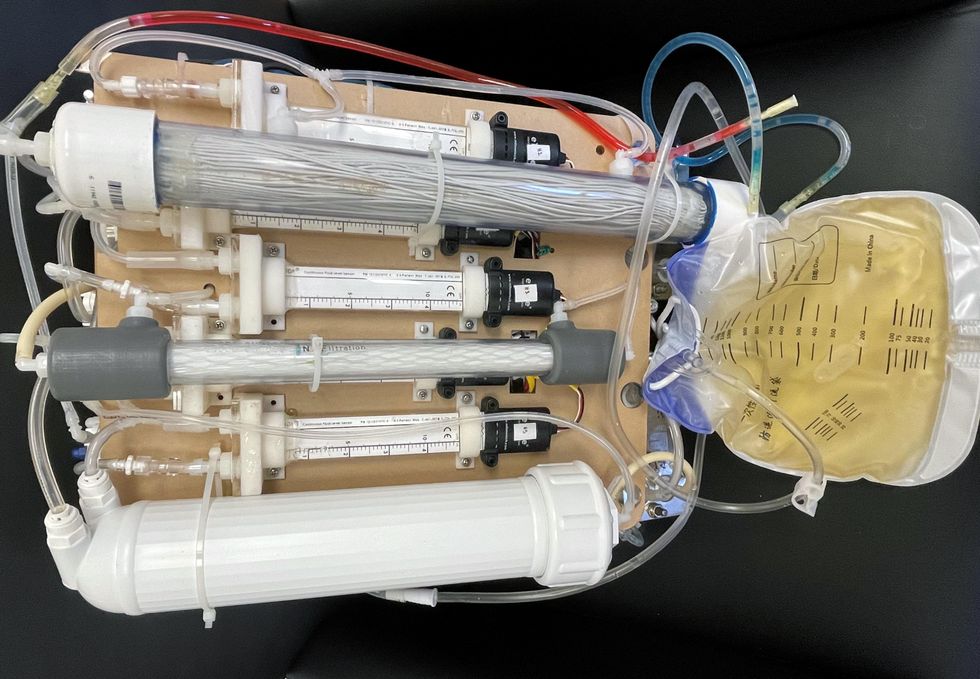
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
New Podcast: Jessica Malaty Rivera Talks Vaccine Hesitancy
"Making Sense of Science" is a monthly podcast that features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This episode is hosted by science and biotech journalist Emily Mullin, summer editor of the award-winning science outlet Leaps.org.
Listen to the episode:
Stacey Khoury felt more fatigued and out of breath than she was used to from just walking up the steps to her job in retail jewelry sales in Nashville, Tennessee. By the time she got home, she was more exhausted than usual, too.
"I just thought I was working too hard and needed more exercise," recalls the native Nashvillian about those days in December 2010. "All of the usual excuses you make when you're not feeling 100%."
As a professional gemologist, being hospitalized during peak holiday sales season wasn't particularly convenient. There was no way around it though when her primary care physician advised Khoury to see a blood disorder oncologist because of her disturbing blood count numbers. As part of a routine medical exam, a complete blood count screens for a variety of diseases and conditions that affect blood cells, such as anemia, infection, inflammation, bleeding disorders and cancer.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before."
While she was in the hospital, a bone marrow biopsy revealed that Khoury had acute myeloid leukemia, or AML, a high-risk blood cancer. After Khoury completed an intense first round of chemotherapy, her oncologist recommended a bone marrow transplant. The potentially curative treatment for blood-cancer patients requires them to first receive a high dose of chemotherapy. Next, an infusion of stem cells from a healthy donor's bone marrow helps form new blood cells to fight off the cancer long-term.
Each year, approximately 8,000 patients in the U.S. with AML and other blood cancers receive a bone marrow transplant from a donor, according to the Center for International Blood and Marrow Transplant Research. But Khoury wasn't so lucky. She ended up being among the estimated 40% of patients eligible for bone marrow transplants who don't receive one, usually because there's no matched donor available.
Khoury's oncologist told her about another option. She could enter a clinical trial for an investigational cell therapy called omidubicel, which is being developed by Israeli biotech company Gamida Cell. The company's cell therapy, which is still experimental, could up a new avenue of treatment for cancer patients who can't get a bone marrow transplant.
Omidubicel consists of stem cells from cord blood that have been expanded using Gamida's technology to ensure there are enough cells for a therapeutic dose. The company's technology allows the immature cord blood cells to multiply quickly in the lab. Like a bone marrow transplant, the goal of the therapy is to make sure the donor cells make their way to the bone marrow and begin producing healthy new cells — a process called engraftment.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before, so there's something very novel and exciting about that," says Ronit Simantov, Gamida Cell's chief medical officer.
Khoury and her husband Rick packed up their car and headed to the closest trial site, the Duke University School of Medicine, roughly 500 miles away. There they met with Mitchell Horowitz, a stem cell transplant specialist at Duke and principal investigator for Gamida's omidubicel study in the U.S.
He told Khoury she was a perfect candidate for the trial, and she enrolled immediately. "When you have one of two decisions, and it's either do this or you're probably not going to be around, it was a pretty easy decision to make, and I am truly thankful for that," she says.
Khoury's treatment started at the end of March 2011, and she was home by July 4 that year. She say the therapy "worked the way the doctors wanted it to work." Khoury's blood counts were rising quicker than the people who had bone marrow matches, and she was discharged from Duke earlier than other patients were.
By expanding the number of cord blood cells — which are typically too few to treat an adult — omidubicel allows doctors to use cord blood for patients who require a transplant but don't have a donor match for bone marrow.
Patients receiving omidubicel first get a blood test to determine their human leukocyte antigen, or HLA, type. This protein is found on most cells in the body and is an important regulator of the immune system. HLA typing is used to match patients to bone marrow and cord blood donors, but cord blood doesn't require as close of a match.
Like bone marrow transplants, one potential complication of omidubicel is graft-versus-host disease, when the donated bone marrow or stem cells register the recipient's body as foreign and attack the body. Depending on the severity of the response, according to the Mayo Clinic, treatment includes medication to suppress the immune system, such as steroids. In clinical trials, the occurrence of graft-versus-host disease with omidubicel was comparable with traditional bone marrow transplants.
"Transplant doctors are working on improving that," Simantov says. "A number of new therapies that specifically address graft-versus-host disease will be making some headway in the coming months and years."
Gamida released the results of the Phase 3 study in February and continues to follow Khoury and the other study patients for their long-term outcomes. The large randomized trial evaluated the safety and efficacy of omidubicel compared to standard umbilical cord blood transplants in patients with blood cancer who didn't have a suitable bone marrow donor. Around 120 patients aged 12 to 65 across the U.S., Europe and Asia were included in the trial. The study found that omidubicel resulted in faster recovery, fewer bacterial and viral infections and fewer days in the hospital.
The company plans to seek FDA approval this year. Simantov anticipates the therapy will receive FDA approval by 2022.
"Opening up cord blood transplants is very important, especially for people of diverse ethnic backgrounds," says oncologist Gary Schiller, principal investigator at the David Geffen School of Medicine at UCLA for Gamida Cell's mid- and late-stage trials. "This expansion technology makes a big difference because it makes cord blood an available option for those who do not have another donor source."
As for Khoury, who proudly celebrated the anniversary of her first transplant in April—she remains cancer free and continues to work full-time as a gemologist. When she has a little free time, she enjoys gardening, sewing, or maybe traveling to national parks like Yellowstone or the Grand Canyon with her husband Rick.