Did researchers finally find a way to lick COVID?

A professor of medicine at the University of Michigan is researching whether lactoferrin, which is found in dairy products such as ice cream, can help to prevent COVID-19 infections.
Already vaccinated and want more protection from COVID-19? A protein found in ice cream could help, some research suggests, though there are a bunch of caveats.
The protein, called lactoferrin, is found in the milk of mammals and thus in dairy products, including ice cream. It has astounding antiviral properties that have been taken for granted and remain largely unexplored because it is a natural product, meaning that it cannot be patented and exploited by pharmaceutical companies.
Still, a few researchers in Europe and elsewhere have sought to better understand the compound.
Jonathan Sexton runs a drug screening program at the University of Michigan where cells are infected with a pathogen and then exposed to a library of the thousands of small molecule drug compounds – which can enter the body more easily than drugs with heavier molecules – approved by the FDA. In addition, the library includes compounds that passed phase 1 safety studies but later proved ineffective against the targeted disease. Each drug is dissolved in a solvent for exposure to the cells in the laborious testing process made feasible by robotic automation.
When COVID hit, researchers scrambled to identify any approved drug that might help fight the infection. Sexton decided to screen the drug library as well as some dietary supplements against SARS-CoV-2, the virus that causes the disease. Sexton says that the grunt work fell to Jesse Wotring, “a very talented PhD student,” who pulled lactoferrin off the shelf. But the regular solvent used in the testing process would destroy the protein, so he had to take another approach and do all the work by hand.
“We were agnostic,” says Sexton, who didn't have a strong interest in lactoferrin or any of the other compounds in the library, but the data was quite clear; lactoferrin “consistently produced the best efficacy...it was the absolute home run.” The findings were published in separate papers last year and in February.
It turns out that lactoferrin has several different mechanisms of action against SARS-CoV-2, inhibiting the virus from entering cells, moving around within them and replicating. Lactoferrin also modulates the overall immune response, which makes it difficult for the virus to simultaneously mutate resistance to the protein at every step of replication. “It has broad efficacy against every [SARS-CoV-2] variant that we've tested,” he says.
From bench to bedside
Sexton's initial interest was to develop a drug for the acute phase of COVID infection, to treat a hospitalized patient or prevent that hospitalization. But with the quick approval of vaccines and drugs to treat the disease, he increasingly focused on ways to better prevent infection and inhibit spread of the virus.
“If you can get lactoferrin to persist in your upper GI tract, then it may very well prevent the primary infection, and that's what we're really interested in.” He reasoned that a chewing gum formula might release enough lactoferrin into the mucosal tissue of the mouth and upper airways to inhibit replication and give the immune system a chance to knock out the virus before it can establish a foothold. It could also reduce the amount of virus spread through talking.
To get enough lactoferrin to have a possible beneficial effect, one would have to drink gallons of milk a day, “and that would have other undesirable consequences, like getting extremely obese,” says Sexton. Obesity is one of the leading risk factors for severe COVID disease.
Testing that theory has been difficult. The easiest way would be a “challenge trial,” where volunteers take the drug, or in this case gum, are exposed to the pathogen, and protection is measured. Some COVID challenge studies have been conducted in Europe but the FDA remains hesitant to allow such a study in the U.S. A traditional prevention study would be like a vaccine trial, involving thousands, perhaps tens of thousands of volunteers over a period of months or years, and it would be very expensive. No one has stepped forward to foot the bill.
So the next step for Sexton is a clinical trial of newly diagnosed COVID patients who will be given standard of care treatment, and layered on top of that they will receive either lactoferrin, probably in pill form, or a placebo. He has identified initial funding. “We would study their viral load over time as well as their symptoms.”
One issue the FDA is grappling with in considering the proposed trial is that it typically decides whether to approve drugs from a factory by applying a rigorous standard, called good manufacturing practices, while food products, which are the source of lactoferrin, are produced under somewhat different standards. The agency still has not finalized rules on how to deal with natural products used as drugs, such as fecal transplants, convalescent plasma, or medical marijuana.
Sexton is frustrated by the delay because lactoferrin derived from bovine milk whey has been used for many decades as a protein supplement by athletes, it is a large component of most infant formula, and the largest number of clinical studies of lactoferrin involve premature infants. There is no question of its safety, he says.
Do it yourself
So what can you do while waiting for regulatory wheels to spin and clinical trial data to be generated?
Could a dose of Ben & Jerry's provide some protection against SARS-CoV-2?
Sexton chuckles at the suggestion. He supposes it couldn't hurt. But to get enough lactoferrin to have a possible beneficial effect, one would have to drink gallons of milk a day, “and that would have other undesirable consequences, like getting extremely obese.” Obesity is one of the leading risk factors for severe COVID disease.
Pseudo-milk products made from soy, almonds, oats, or other plant products do not contain lactoferrin; it has to come from a teat. So that rules them out.
Whey-based protein shakes might be a useful way to add lactoferrin to the diet.
Probably the best option is to take conventional gelatin capsules of lactoferrin that are widely available wherever supplements are sold. Sexton calculates that about a gram a day, four 250 milligram capsules, should do it. He advises two in the morning and two a night. “You really want to take them on an empty stomach...your stomach treats [the lactoferrin protein] like it would a steak” and chops it for absorption in the intestine, which you do not want. About 70 percent of lactoferrin can get through an empty stomach, but eating food cranks up digestive gastric acids and the amount of intact lactoferrin that gets through to the gut plummets.
Sexton cautions, “We have not determined clinical efficacy yet,” and he is not offering advice as a physician, but in the spirit of harm reduction, he realizes that some people are going to try things that might help them. Lactoferrin “is remarkably safe. And so people have to make their own decisions about what they are willing to take and what they are not,” he says.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
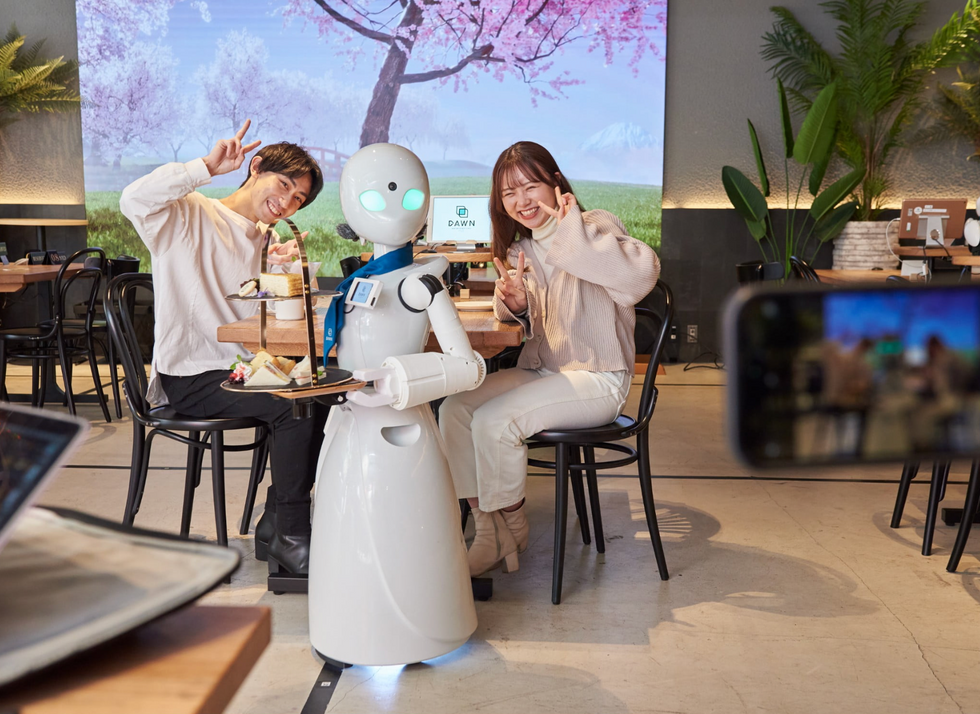
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
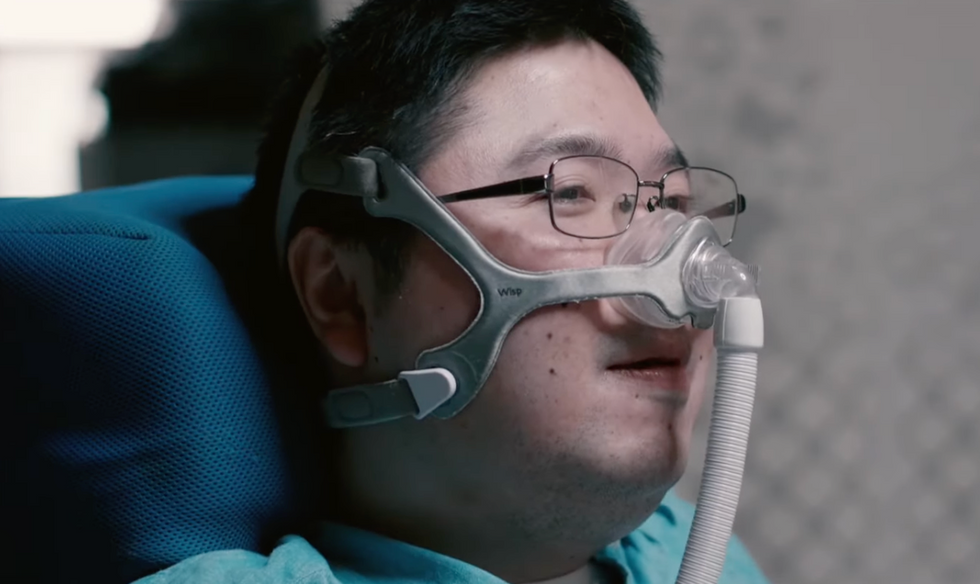
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

