A vaccine for Lyme disease could be coming. But will patients accept it?

Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
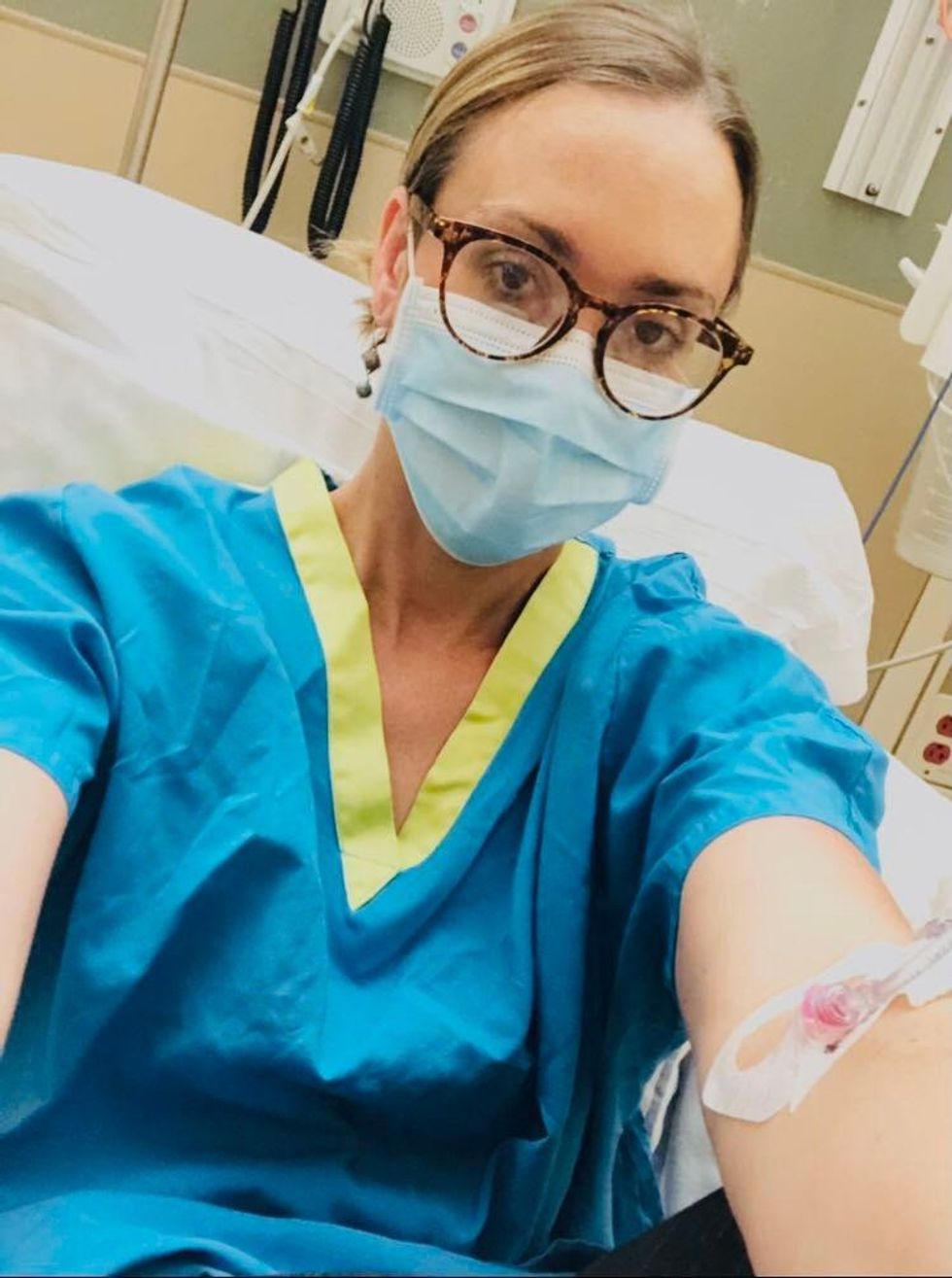
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”
They received retinal implants to restore their vision. Then the company turned its back on them.
A company called Second Sight made an implant that partially restored vision to people who'd been blind for decades. But when Second Sight pivoted, it stopped servicing its product, leaving many in the dark.
The first thing Jeroen Perk saw after he partially regained his sight nearly a decade ago was the outline of his guide dog Pedro.
“There was a white floor, and the dog was black,” recalls Perk, a 43-year-old investigator for the Dutch customs service. “I was crying. It was a very nice moment.”
Perk was diagnosed with retinitis pigmentosa as a child and had been blind since early adulthood. He has been able to use the implant placed into his retina in 2013 to help identify street crossings, and even ski and pursue archery. A video posted by the company that designed and manufactured the device indicates he’s a good shot.
Less black-and-white has been the journey Perk and others have been on after they were implanted with the Argus II, a second-generation device created by a Los Angeles-based company called Second Sight Medical Devices.
The Argus II uses the implant and a video camera embedded in a special pair of glasses to provide limited vision to those with retinitis pigmentosa, a genetic disease that causes cells in the retina to deteriorate. The camera feeds information to the implant, which sends electrical impulses into the retina to recapitulate what the camera sees. The impulses appear in the Argus II as a 60-pixel grid of blacks, grays and whites in the user’s eye that can render rough outlines of objects and their motion.
Smartphone and computer manufacturers typically stop issuing software upgrades to their devices after two or three years, eventually rendering them bricks. But is the smartphone approach acceptable for a device that helps restore the most crucial sense a human being possesses?
Ross Doerr, a retired disability rights attorney in Maine who received an Argus II in 2019, describes the field of vision as the equivalent of an index card held at arm’s length. Perk often brings objects close to his face to decipher them. Moreover, users must swivel their heads to take in visual data; moving their eyeballs does not work.
Despite its limitations, the Argus II beats the alternative. Perk no longer relies on his guide dog. Doerr was uplifted when he was able to see the outlines of Christmas trees at a holiday show.
“The fairy godmother department sort of reaches out and taps you on the shoulder once in a while,” Doerr says of his implant, which came about purely by chance. A surgeon treating his cataracts was partnered with the son of another surgeon who was implanting the devices, and he was referred.
Doerr had no reason to believe the shower of fairy dust wouldn’t continue. Second Sight held out promises that the Argus II recipients’ vision would gradually improve through upgrades to much higher pixel densities. The ability to recognize individual faces was even touted as a possibility. In the winter of 2020, Doerr was preparing to travel across the U.S. to Second Sight’s headquarters to receive an upgrade. But then COVID-19 descended, and the trip was canceled.
The pandemic also hit Second Sight’s bottom line. Doerr found out about its tribulations only from one of the company’s vision therapists, who told him the entire department was being laid off. Second Sight cut nearly 80% of its workforce in March 2020 and announced it would wind down operations.

Ross Doerr has mostly stopped using his Argus II, the result of combination of fear of losing its assistance from wear and tear and disdain for the company that brought it to market.
Jan Doerr
Second Sight’s implosion left some 350 Argus recipients in the metaphorical dark about what to do if their implants failed. Skeleton staff seem to have rarely responded to queries from their customers, at least based on the experiences of Perk and Doerr. And some recipients have unfortunately returned to the actual dark as well, as reports have surfaced of Argus II failures due to aging or worn-down parts.
Product support for complex products is remarkably uneven. Although the iconic Ford Mustang ceased production in the late 1960s, its parts market is so robust that it’s theoretically possible to assemble a new vehicle from recently crafted components. Conversely, smartphone and computer manufacturers typically stop issuing software upgrades to their devices after two or three years, eventually rendering them bricks. Consumers have accepted both extremes.
But is the smartphone approach acceptable for a device that helps restore the most crucial sense a human being possesses?
Margaret McLean, a senior fellow at the Markkula Center for Applied Ethics at Santa Clara University in California, notes companies like Second Sight have a greater obligation for product support than other consumer product ventures.
“In this particular case, you have a great deal of risk that is involved in using this device, the implant, and the after care of this device,” she says. “You cannot, like with your car, decide that ‘I don’t like my Mustang anymore,’ and go out and buy a Corvette.”
And, whether the Argus II implant works or not, its physical presence can impact critical medical decisions. Doerr’s doctor wanted him to undergo an MRI to assist in diagnosing attacks of vertigo. But the physician was concerned his implant might interfere. With the latest available manufacturer advisories on his implant nearly a decade old, the procedure was held up. Doerr spent months importuning Second Sight through phone calls, emails and Facebook postings to learn if his implant was contraindicated with MRIs, which he never received. Although the cause of his vertigo was found without an MRI, Doerr was hardly assured.
“Put that into context for a minute. I get into a serious car accident. I end up in the emergency room, and I have a tag saying I have an implanted medical device,” he says. “You can’t do an MRI until you get the proper information from the company. Who’s going to answer the phone?”
Second Sight’s management did answer the call to revamp its business. It netted nearly $78 million through a private stock placement and an initial public offering last year. At the end of 2021, Second Sight had nearly $70 million in cash on hand, according to a recent filing with the Securities and Exchange Commission.
And while the Argus II is still touted at length on Second Sight’s home page, it appears little of its corporate coffers are earmarked toward its support. These days, the company is focused on obtaining federal approvals for Orion, a new implant that would go directly into the recipient’s brain and could be used to remedy blindness from a variety of causes. It obtained a $6.4 million grant from the National Institutes of Health in May 2021 to help develop Orion.
Presented with a list of written questions by email, Second Sight’s spokesperson, Dave Gentry of the investor relations firm Red Chip Companies, copied a subordinate with an abrupt message to “please handle.” That was the only response from a company representative. A call to Second Sight acting chief executive officer Scott Dunbar went unreturned.
Whether or not the Orion succeeds remains to be seen. The company’s SEC filings suggest a viable and FDA-approved device is years away, and that operational losses are expected for the “foreseeable future.” Second Sight reported zero revenue in 2020 or 2021.
Moreover, the experiences of the Argus II recipients could color the reception of future Second Sight products. Doerr notes that his insurer paid nearly $500,000 to implant his device and for training on how to use it.
“What’s the insurance industry going to say the next time this crops up?” Doerr asks, noting that the company’s reputation is “completely shot” with the recipients of its implants.
Perk, who made speeches to praise the Argus II and is still featured in a video on the Second Sight website, says he also no longer supports the company.

Jeroen Perk, an investigator for the Dutch customs service, cried for joy after partially regaining his sight, but he no longer trusts Second Sight, the company that provided his implant.
Nanda Perk
Nevertheless, Perk remains highly reliant on the technology. When he dropped an external component of his device in late 2020 and it broke, Perk briefly debated whether to remain blind or find a way to get his Argus II working again. Three months later, he was able to revive it by crowdsourcing parts, primarily from surgeons with spare components or other Argus II recipients who no longer use their devices. Perk now has several spare parts in reserve in case of future breakdowns.
Despite the frantic efforts to retain what little sight he has, Perk has no regrets about having the device implanted. And while he no longer trusts Second Sight, he is looking forward to possibly obtaining more advanced implants from companies in the Netherlands and Australia working on their own products.
Doerr suggests that biotech firms whose implants are distributed globally be bound to some sort of international treaty requiring them to service their products in perpetuity. Such treaties are still applied to the salvage rights for ships that sunk centuries ago, he notes.
“I think that in a global tech economy, that would be a good thing,” says McLean, the fellow at Santa Clara, “but I am not optimistic about it in the near term. Business incentives push toward return on share to stockholders, not to patients and other stakeholders. We likely need to rely on some combination of corporately responsibility…and [international] government regulation. It’s tough—the Paris Climate Accord implementation at a slow walk comes to mind.”
Unlike Perk, Doerr has mostly stopped using his Argus II, the result of combination of fear of losing its assistance from wear and tear and disdain for the company that brought it to market. At 70, Doerr says he does not have the time or energy to hold the company more accountable. And with Second Sight having gone through a considerable corporate reorganization, Doerr believes a lawsuit to compel it to better serve its Argus recipients would be nothing but an extremely costly longshot.
“It’s corporate America at its best,” he observes.
Rehabilitating psychedelic drugs: Another key to treating severe mental health disorders
A recent review paper found evidence that using psychedelics such as MDMA can help with treating a variety of common mental illnesses, but experts fear that research might easily be shut down in the future.
Lori Tipton's life was a cascade of trauma that even a soap opera would not dare inflict upon a character: a mentally unstable family; a brother who died of a drug overdose; the shocking discovery of the bodies of two persons her mother had killed before turning the gun on herself; the devastation of Hurricane Katrina that savaged her hometown of New Orleans; being raped by someone she trusted; and having an abortion. She suffered from severe PTSD.
“My life was filled with anxiety and hypervigilance,” she says. “I was constantly afraid and had mood swings, panic attacks, insomnia, intrusive thoughts and suicidal ideation. I tried to take my life more than once.” She was fortunate to be able to access multiple mental health services, “And while at times some of these modalities would relieve the symptoms, nothing really lasted and nothing really address the core trauma.”
Then in 2018 Tipton enrolled in a clinical trial that combined intense sessions of psychotherapy with limited use of Methylenedioxymethamphetamine, or MDMA, a drug classified as a psychedelic and commonly known as ecstasy or Molly. The regimen was arduous; 1-2 hour preparation sessions, three sessions where MDMA was used, which lasted 6-8 hours, and lengthy sessions afterward to process and integrate the experiences. Two therapists were with her every moment of the three-month program that totaled more than 40 hours.
“It was clear to me that [the therapists] weren't going to heal me, that I was going to have to do the work for myself, but that they were there to completely support my process,” she says. “But the effects of MDMA were really undeniable for me. I felt embodied in a way that I hadn't in years. PTSD had robbed me of the ability to feel safe in my own body.”
Tipton doesn’t think the therapy completely cured her PTSD. “But when I completed the trial in 2018, I no longer qualified for the diagnosis, and I still don't qualify for the diagnosis today,” she told an April workshop on psychedelics as mental health treatment by the National Academies of Sciences, Engineering and Medicine, or NASEM.
A Champion
Rick Doblin has been a catalyst behind much of the contemporary research into psychedelics. Prior to the DEA clamp down, the Boston psychotherapist had seen that MDMA and other psychedelics could benefit some of his patients where other measures had failed. He immediately organized efforts to question the drug rescheduling but to little avail. In 1986, he created the nonprofit Multidisciplinary Association for Psychedelic Studies (MAPS), which slowly laid the scientific foundation for clinical trials, including the one that Tipton joined, using psychedelics to treat mental health conditions.
Now, only slowly, have researchers been able to explore the power of these drugs to treat a broad spectrum of severely debilitating mental health conditions, including trauma, depression, and PTSD, where other available treatments proved inadequate.
“Psychedelic psychotherapy is an attempt to go after the root causes of the problems with just a relatively few administrations, as contrasted to most of the psychiatric drugs used today that are mostly just reducing symptoms and are meant to be taken on a daily basis,” Doblin said in a 2019 TED Talk. Most of these drugs can have broad effect but “some are probably more effective than others for certain conditions,” he added in a recent interview with Leaps.org. Comparative head-to-head studies of psychedelic therapies simply have not been conducted.
Their mechanisms of action are poorly understood and can vary between drugs, but it is generally believed that psychedelics change the activity of neurons so that the brain processes information differently, says Katrin Preller, a neuropsychologist at the University of Zurich. A recent important study in Nature Medicine by Richard Daws and colleagues used functional magnetic resonance imaging (fMRI) of the brain and found that “functional networks became more functionally interconnected and flexible after psilocybin treatment…implying that psilocybin's antidepressant action may depend on a global increase in brain network integration.”
Rosalind Watts, a clinical investigator at the Imperial College in London, believes there is “an overestimation of the importance of the drug and an underestimation of the importance of the [therapeutic] context” in psychedelic research. “It is unethical to provide the drug without the other,” she says. Doblin notes that “psychotherapy outcomes research demonstrates that the therapeutic alliance between the therapist and the patients is the single most predictive factor of outcomes. [It is] trust and the sense of safety, the willingness to go into difficult spaces” that makes clinical breakthroughs possible with the drug.
Excitement and Challenges
Recurrent themes expressed at the NASEM workshop were exciting glimpses of the potential for psychedelics to treat mental health conditions combined with the challenges of realizing those potentials. A recent review paper found evidence that using psychedelics can help with treating a variety of common mental illnesses, but the paper could identify only 14 clinical trials of classic psychedelics published since 1991. Much of the reason is that the drugs are not patentable and so the pharmaceutical industry has no interest in investing in expensive clinical trials to bring them to market. MAPS has raised about $135 million over its 36-year history to conduct such research, says Doblin, the vast majority of it from individual donors and none from foundations.
The workshop participants’ views also were colored by the history of drug crackdowns and a fear that research might easily be shut down in the future. There was great concern that use of psychedelics should be confined to clinical trials with high safety and ethical standards, instead of doctors and patients experimenting on their own. “We need to get it right this time,” says Charles Grob, a psychiatrist at the UCLA School of Medicine. But restricting access to psychedelics will become even more difficult now that Oregon and several cities have acted to decriminalize possession and use of many of these drugs.
The experience with ketamine also troubled Grob. He is hoping to “mitigate the rush of rapid commercialization” that occurred with that drug. Ketamine technically is not a psychedelic though it does share some of their potentially euphoric properties. In 2019, soon after the FDA approved a form of ketamine with a limited label indication to treat depression, for profit clinics sprang up promoting off label use of the drug for psychiatric conditions where there was little clinical evidence of efficacy. He fears the same thing will happen when true psychedelics are made available.
If these therapies are approved, access to them is likely to be a problem. The drugs themselves are cheap but the accompanying therapy is not, and there is a shortage of trained psychotherapists. Mental health services often are not adequately covered by health insurance, while the poor and people of color suffer additional burdens of inadequate access. Doblin is committed to health care equity by training additional providers and by investigating whether some of the preparatory and integration sessions might be handled in a group setting. He says it is important that the legal aspects of psychedelics also be addressed so that patients “don't have to go underground” in order to receive this care.

