A vaccine for Lyme disease could be coming. But will patients accept it?

Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
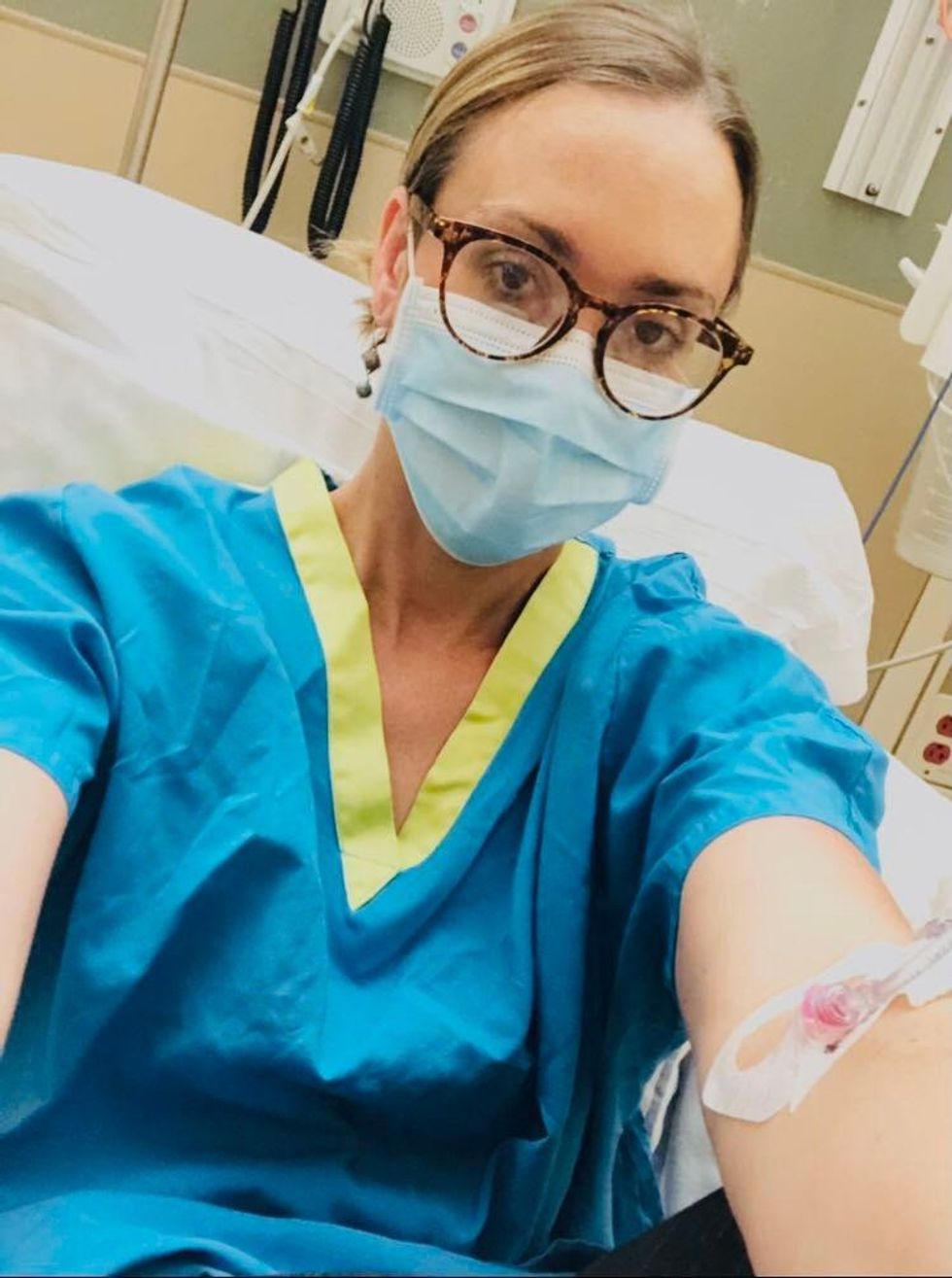
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”
Dr. Andrew Brooks of RUCDR Infinite Biologics holds up a saliva sample.
The patient tilts back her head and winces as the long swab stick pushes six inches up her nose. The tip twirls around uncomfortably before it's withdrawn.
"Our saliva test can detect the virus in asymptomatic and pre-symptomatic cases."
A gloved and gowned healthcare worker wearing a face shield and mask tells the patient that she will learn whether she is positive for COVID-19 as soon as the lab can process her test.
This is the typical unpleasant scenario for getting a coronavirus test. But times are rapidly changing: Today, for the first time, the U.S. Food and Drug Administration cleared one company to sell saliva collection kits for individuals to use at home.
Scientists at the startup venture, RUCDR Infinite Biologics at Rutgers University in New Jersey, say that saliva testing offers an easier, more useful alternative to the standard nasal swab.
"Our saliva test can detect the virus in asymptomatic and pre-symptomatic cases," said Dr. Andrew Brooks, chief operating officer at RUCDR.
Another venture, Darwin BioSciences in Colorado, has separately developed an innovative method of testing saliva for the coronavirus that causes COVID-19.
Saliva testing can allow earlier detection to identify people who may not know they are contagious, say scientists at both companies. In addition, because patients spit into a tube or cup, saliva testing is safer for healthcare workers than taking swabs. This frees up scarce personal protective equipment (PPE) for use elsewhere. Nasal swabs themselves have been in scarce supply.
Saliva testing, if it becomes widespread, potentially could mean opening society sooner. The more ubiquitous testing becomes across the population, experts say, the more feasible it becomes for public health officials to trace and isolate contacts, especially of asymptomatic cases. Testing early and often will be essential to containing emerging hot spots before a vast outbreak can take root.
Darwin Biosceiences is preparing to seek an FDA Emergency Use Authorization (EUA) this month for its patented "CoVScreen" testing system, which potentially could be available to labs nationally by mid-summer.
Meanwhile, Infinite Biologics will now begin selling kits to consumers for home collection, upon order by a physician. The FDA said that the company's saliva test was as accurate as the nasal swab method used by health care professionals. An FDA summary documenting the company's data reported: "There was 100% positive and negative agreement between the results obtained from testing of saliva and those obtained from nasopharyngeal and oropharyngeal swabs."
The greatest scientific advantage, said Dr. Brooks, is that nasal and oral swabs only collect the surface area where the swab goes, which may not be the place with most viral load. In contrast, the virus occurs throughout a saliva sample, so the test is more trustworthy.
The lab at Rutgers can process 20,000 tests a day, with a 48-hour turnaround. They have 75,000 tests ready to ship now.
The Leap: Detecting Sickness Before You Feel It
"We wanted to create a device that could detect infections before symptoms appeared," explained Nicholas Meyerson, co-founder and CEO of Darwin.
For more than 300 years, he said, "the thermometer was the gold standard for detecting disease because we thought the first sign of illness was a fever. This COVID-19 pandemic has proven that not all pathogens cause a fever. You can be highly contagious without knowing it."
"The question is whether we can scale up fast enough to meet the need. I believe saliva testing can help."
Therefore, Meyerson and co-founder Sara Sawyer from the University of Colorado began to identify RNA biomarkers that can sense when a pathogen first enters a molecule and "sets off alarms." They focused on the nucleic acids concentrated in saliva as the best and easiest place to collect samples for testing.
"The isothermal reaction in saliva takes place at body or room temperature," he said, "so there's no need for complicated testing machinery. The chemical reaction can be read out on a paper strip, like a pregnancy test -- two stripes if you're sick, and one stripe if you're okay."
Before the pandemic, limited but successful human trials were already underway at CU in Boulder and at the CU Anschutz Medical Campus east of Denver. "This was our proof of concept," he said.
Darwin was founded in March and has secured enough venture capital to concentrate protype development on detecting the virus causing COVID-19. So far, said Meyerson, "Everything works."
A small double-blind test of 30 samples at CU produced 100 percent accuracy. "I'm not sure if that will hold true as we go into clinical trials," he said, "but I'm confident we will satisfy all the requirements for at least 95 percent clinical validation."
The specific "CoVStick" test strips will roll out soon, he said: "We hope before the second wave of the pandemic hits."
The broader saliva test-strip product from Darwin, "SickStick," is still one to two years away from deployment by the military and introduction into the consumer drugstore market for home use, said Meyerson. It will affordably and quickly detect a range of viral and bacterial infections.

An illustration of the "CoVStick."
(Darwin Biosciences)
A Potential Game Changer
Society needs widespread testing daily, said George Church, founding core faculty of the Wyss Institute for Biologically Inspired Engineering at Harvard University. Speaking at an online SynBioBeta webinar in April, he urged developing stockpiles of testing kits for home use.
As for any potential of false positives, Church said a much bigger risk is not having enough tests.
"Saliva testing is going to speed up the timeline for opening society a lot," said Meyerson. "People need to self-collect samples at home. A lot more people are going to be willing to spit into a tube than to push a swab six inches up their own nose."
Brooks, of Rutgers, addressed the big picture. "It's critical that we open society as soon as possible to minimize the economic impact of the pandemic. Testing is the surest and safest path. The question is whether we can scale up fast enough to meet the need. I believe saliva testing can help."
A vaccine from Moderna could be available as early as January, Fauci recently said.
Earlier this year, biotech company Moderna broke world records for speed in vaccine development. Their researchers translated the genetic code of the coronavirus into a vaccine candidate in just 42 days.
We're about to expand our safety data in Phase II.
Phase I of the clinical trial started in Seattle on March 16th, with the already-iconic image of volunteer Jennifer Haller calmly receiving the very first dose.
Instead of traditional methods, this vaccine uses a new -- and so far unproven -- technology based on synthetic biology: It hijacks the software of life – messenger RNA – to deliver a copy of the virus's genetic sequence into cells, which, in theory, triggers the body to produce antibodies to fight off a coronavirus infection.
U.S. National Institute of Allergy and Infectious Diseases Director Anthony Fauci called the vaccine's preclinical data "impressive" and told National Geographic this week that a vaccine could be ready for general use as early as January.
The Phase I trial has dosed 45 healthy adults. Phase II trials are about to start, enrolling around 600 adults. Pivotal efficacy trials would follow soon thereafter, bankrolled in collaboration with the government office BARDA (Biomedical Advanced Research and Development Authority).
Today, the chief medical officer of Moderna, Tal Zaks, answered burning questions from the public in a webinar hosted by STAT. Here's an edited and condensed summary of his answers.
1) When will a vaccine become available?
We expect to have data in early summer about the antibody levels from our mRNA vaccine. At the same time, we can measure the antibody levels of people who have had the disease, and we should be able to measure the ability of those antibodies to prevent disease.
We will not yet know if the mRNA vaccine works to prevent disease, but we could soon talk about a potential for benefit. We don't yet know about risk. We're about to expand our safety data in Phase II.
In the summer, there is an expectation that we will be launching pivotal trials, in collaboration with government agencies that are helping fund the research. The trials would be launched with the vaccine vs. a placebo with the goal of establishing: How many cases can we show we prevented with the vaccine?
This is determined by two factors: How big is the trial? And what's the attack rate in the population we vaccinate? The challenge will be to vaccinate in the areas where the risk of infection is still high in the coming months, and we're able to vaccinate and demonstrate fewer infections compared to a placebo. If the disease is happening faster in a given area, you will be able to see an outcome faster. Potentially by the end of the year, we will have the data to say if the vaccine works.
Will that be enough for regulatory approval? The main question is: When will we cross the threshold for the anticipated benefit of a presumed vaccine to be worth the risk?
There is a distinction between approval for those who need it most, like the elderly. Their unmet need and risk/benefit is not the same as it is for younger adults.
My private opinion: I don't think it's a one-size-fits-all. It will be a more measured stance.
2) Can you speed up the testing process with challenge studies, where volunteers willingly get infected?
It's a great question and I applaud the people who ask it and I applaud those signing up to do it. I'm not sure I am a huge fan, for both practical and ethical reasons. The devil is in the details. A challenge study has to show us a vaccine can prevent not just infection but prevent disease. Otherwise, how do I know the dose in the challenge study is the right dose? If you take 100 young people, 90 of them will get mild or no disease. Ten may end up in hospital and one in the ICU.
Also, the timeline. Can it let you skip Phase II of large efficacy trial? The reality for us is that we are about to start Phase II anyway. It would be months before a challenge trial could be designed. And ethically: everybody agrees there is a risk that is not zero of having very serious disease. To justify the risk, we have to be sure the benefit is worth it - that it actually shrunk the timeline. To just give us another data point, I find it hard to accept.
This technology allows us to scale up manufacturing and production.
3) What was seen preclinically in the animal models with Moderna's mRNA vaccines?
We have taken vaccines using our technology against eight different viruses, including two flu strains. In every case, in the preclinical model, we showed we could prevent disease, and when we got to antibody levels, we got the data we wanted to see. In doses of 25-100 micrograms, that usually ends up being a sweet spot where we see an effect. It's a good place as to the expectation of what we will see in Phase I trials.
4) Why is Moderna pursuing an mRNA virus instead of a traditional inactivated virus or recombinant one? This is an untried technology.
First, speed matters in a pandemic. If you have tech that can move much quicker, that makes a difference. The reason we have broken world records is that we have invested time and effort to be ready. We're starting from a platform where it's all based on synthetic biology.
Second, it's fundamental biology - we do not need to make an elaborate vaccine or stick a new virus in an old virus, or try to make a neutralizing but not binding virus. Our technology is basically mimicking the virus. All life works on making proteins through RNA. We have a biological advantage by teaching the immune system to do the right thing.
Third, this technology allows us to scale up manufacturing and production. We as a company have always seen this ahead of us. We invested in our own manufacturing facility two years ago. We have already envisioned scale up on two dimensions. Lot size and vaccines. Vaccines is the easier piece of it. If everybody gets 100 micrograms, it's not a heck of a lot. Prior to COVID, our lead program was a CMV (Cytomegalovirus) vaccine. We had envisioned launching Phase III next year. We had been already well on the path to scale up when COVID-19 caught us by surprise. This would be millions and millions of doses, but the train tracks have been laid.
5) People tend to think of vaccines as an on-off switch -- you get a vaccine and you're protected. But efficacy can be low or high (like the flu vs. measles vaccines). How good is good enough here for protection, and could we need several doses?
Probably around 50-60 percent efficacy is good enough for preventing a significant amount of disease and decreasing the R0. We will aim higher, but it's hard to estimate what degree of efficacy to prepare for until we do the trial. (For comparison, the average flu vaccine efficacy is around 50 percent.)
We anticipate a prime boost. If our immune system has never seen a virus, you can show you're getting to a certain antibody level and then remind the immune system (with another dose). A prime boost is optimal.
My only two competitors are the virus and the clock.
6) How would mutations affect a vaccine?
Coronaviruses tend to mutate the least compared to other viruses but it's entirely possible that it mutates. The report this week about those projected mutations on the spike protein have not been predicted to alter the critical antibodies.
As we scale up manufacturing, the ability to plug in a new genetic sequence and get a new vaccine out there will be very rapid.
For flu vaccine, we don't prove efficacy every year. If we get to the same place with an mRNA vaccine, we will just change the sequence and come out with a new vaccine. The path to approval would be much faster if we leverage the totality of efficacy data like we do for flu.
7) Will there be more than one vaccine and how will they be made available?
I hope so, I don't know. The path to making these available will go through a public-private partnership. It's not your typical commercial way of deploying a vaccine. But my only two competitors are the virus and the clock. We need everybody to be successful.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.