Masks and Distancing Won't Be Enough to Prevent School Outbreaks, Latest Science Suggests

The likely dominant mode of aerosol transmission cannot be ignored in school settings.
Never has the prospect of "back to school" seemed so ominous as it does in 2020. As the number of COVID-19 cases climb steadily in nearly every state, the prospect of in-person classes are filling students, parents, and faculty alike with a corresponding sense of dread.
The notion that children are immune or resistant to SARS-CoV-2 is demonstrably untrue.
The decision to resume classes at primary, secondary, and collegiate levels is not one that should be regarded lightly, particularly as coronavirus cases skyrocket across the United States.
What should be a measured, data-driven discussion that weighs risks and benefits has been derailed by political talking points. President Trump has been steadily advocating for an unfettered return to the classroom, often through imperative "OPEN THE SCHOOLS!!!" tweets. In July, Secretary of Education Betsy DeVos threatened to withhold funding from schools that did not reopen for full-time, in-person classes, despite not having the authority to do so. Like so many public health issues, opening schools in the midst of a generational pandemic has been politicized to the point that the question of whether it is safe to do so has been obscured and confounded. However, this question still deserves to be examined based on evidence.
What We Know About Kids and COVID-19
Some arguments for returning to in-person education have focused on the fact that children and young adults are less susceptible to severe disease. In some cases, people have stated that children cannot be infected, pointing to countries that have resumed in-person education with no associated outbreaks. However, those countries had extremely low community transmission and robust testing and surveillance.
The notion that children are immune or resistant to SARS-CoV-2 is demonstrably untrue: children can be infected, they can become sick, and, in rare cases, they can die. Children can also transmit the virus to others, especially if they are in prolonged proximity to them. A Georgia sleepaway camp was the site of at least 260 cases among mostly children and teenagers, some as young as 6 years old. Children have been shown to shed infectious virus in their nasal secretions and have viral loads comparable to adults. Children can unquestionably be infected with SARS-CoV-2 and spread it to others.
The more data emerges, the more it appears that both primary and secondary schools and universities alike are conducive environments for super-spreading. Mitigating these risks depends heavily on individual schools' ability to enforce reduction measures. So far, the evidence demonstrates that in most cases, schools are unable to adequately protect students or staff. A school superintendent from a small district in Arizona recently described an outbreak that occurred among staff prior to in-person classes resuming. Schools that have opened so far have almost immediately reported new clusters of cases among students or staff.
This is because it is impossible to completely eliminate risk even with the most thoughtful mitigation measures when community transmission is high. Risk can be reduced, but the greater the likelihood that someone will be exposed in the community, the greater the risk they might pass the virus to others on campus or in the classroom.
There are still many unknowns about SARS-CoV-2 transmission, but some environments are known risks for virus transmission: enclosed spaces with crowds of people in close proximity over extended durations. Transmission is thought to occur predominantly through inhaled aerosols or droplets containing SARS-CoV-2, which are produced through common school activities such as breathing, speaking, or singing. Masks reduce but do not eliminate the production of these aerosols. Implementing universal mask-wearing and physical distancing guidelines will furthermore be extraordinarily challenging for very young children.
Smaller particle aerosols can remain suspended in the air and accumulate over time. In an enclosed space where people are gathering, such as a classroom, this renders risk mitigation measures such as physical distancing and masks ineffective. Many classrooms at all levels of education are not conducive to improving ventilation through low-cost measures such as opening windows, much less installing costly air filtration systems.
As a risk reduction measure, ventilation greatly depends on factors like window placement, window type, room size, room occupancy, building HVAC systems, and overall airflow. There isn't much hard data on the specific effects of ventilation on virus transmission, and the models that support ventilation rely on assumptions based on scant experimental evidence that doesn't account for virologic parameters.
There is also no data about how effective air filtration or UV systems would be for SARS-CoV-2 transmission risk reduction, so it's hard to say if this would result in a meaningful risk reduction or not. We don't have enough data outside of a hospital setting to support that ventilation and/or filtration would significantly reduce risk, and it's impractical (and most likely impossible in most schools) to implement hospital ventilation systems, which would likely require massive remodeling of existing HVAC infrastructure. In a close contact situation, the risk reduction might be minimal anyway since it's difficult to avoid exposure to respiratory aerosols and droplets a person is exhaling.
You'd need to get very low rates in the local community to open safely in person regardless of other risk reduction measures, and this would need to be complemented by robust testing and contact tracing capacity.
Efforts to resume in-person education depend heavily on school health and safety plans, which often rely on self-reporting of symptoms due to insufficient testing capacity. Self-reporting is notoriously unreliable, and furthermore, SARS-CoV-2 can be readily transmitted by pre-symptomatic individuals who may be unaware that they are sick, making testing an essential component of any such plan. Primary and secondary schools are faced with limited access to testing and no funds to support it. Even in institutions that include a testing component in their reopening plans, this is still too infrequent to support the full student body returning to campus.
Economic Conflicts of Interest
Rebecca Harrison, a PhD candidate at Cornell University serving on the campus reopening committee, is concerned that her institution's plan places too much faith in testing capacity and is over-reliant on untested models. Harrison says that, as a result, students are being implicitly encouraged to return to campus and "very little has been done to actively encourage students who are safe and able to stay home, to actually stay home."
Harrison also is concerned that her institution "presumably hopes to draw students back from the safety of their parents' basements to (re)join the residential campus experience ... and drive revenue." This is a legitimate concern. Some schools may be actively thwarting safety plans in place to protect students based on financial incentives. Student athletes at Colorado State have alleged that football coaches told them not to report COVID-19 symptoms and are manipulating contact tracing reports.
Public primary and secondary schools are not dependent on student athletics for revenue, but nonetheless are susceptible to state and federal policies that tie reopening to budgets. If schools are forced to make decisions based on a balance sheet, rather than the health and safety of students, teachers, and staff, they will implement health and safety plans that are inadequate. Schools will become ground zero for new clusters of cases.
Looking Ahead: When Will Schools Be Able to Open Again?
One crucial measure is the percent positivity rate in the local community, the number of positive tests based on all the tests that are done. Some states, like California, have implemented policies guiding the reopening of schools that depend in part on a local community's percent positivity rate falling under 8 percent, among other benchmarks including the rate of new daily cases. Currently, statewide, test positivity is below 7%, with an average of 3 new daily cases per 1000 people per day. However, the California department of health acknowledges that new cases per day are underreported. There are 6.3 million students in the California public school system, suggesting that at any given time, there could be nearly 20,000 students who might be contagious, without accounting for presymptomatic teachers and staff. In the classroom environment, just one of those positive cases could spread the virus to many people in one day despite masks, distancing, and ventilation.
You'd need to get very low rates in the local community to open safely in person regardless of other risk reduction measures, and this would need to be complemented by robust testing and contact tracing capacity. Only with rapid identification and isolation of new cases, followed by contact tracing and quarantine, can we break chains of transmission and prevent further spread in the school and the larger community.
None of these safety concerns diminish the many harms associated with the sudden and haphazard way remote learning has been implemented. Online education has not been effective in many cases and is difficult to implement equitably. Young children, in particular, are deprived of the essential social and intellectual development they would normally get in a classroom with teachers and their peers. Parents of young children are equally unprepared and unable to provide full-time instruction. Our federal leadership's catastrophic failure to contain the pandemic like other countries has put us in this terrible position, where we must choose between learning or spreading a deadly pathogen.
Blame aside, parents, educators, and administrators must decide whether to resume in-person classes this fall. Those decisions should be based on evidence, not on politics or economics. The data clearly shows that community transmission is out of control throughout most of the country. Thus, we ignore the risk of school outbreaks at our peril.
[Editor's Note: Here's the other essay in the Back to School series: 5 Key Questions to Consider Before Sending Your Child Back to School.]
Scientists have known about and studied heart rate variability, or HRV, for a long time and, in recent years, monitors have come to market that can measure HRV accurately.
This episode is about a health metric you may not have heard of before: heart rate variability, or HRV. This refers to the small changes in the length of time between each of your heart beats.
Scientists have known about and studied HRV for a long time. In recent years, though, new monitors have come to market that can measure HRV accurately whenever you want.
Five months ago, I got interested in HRV as a more scientific approach to finding the lifestyle changes that work best for me as an individual. It's at the convergence of some important trends in health right now, such as health tech, precision health and the holistic approach in systems biology, which recognizes how interactions among different parts of the body are key to health.
But HRV is just one of many numbers worth paying attention to. For this episode of Making Sense of Science, I spoke with psychologist Dr. Leah Lagos; Dr. Jessilyn Dunn, assistant professor in biomedical engineering at Duke; and Jason Moore, the CEO of Spren and an app called Elite HRV. We talked about what HRV is, research on its benefits, how to measure it, whether it can be used to make improvements in health, and what researchers still need to learn about HRV.
*Talk to your doctor before trying anything discussed in this episode related to HRV and lifestyle changes to raise it.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Show notes
Spren - https://www.spren.com/
Elite HRV - https://elitehrv.com/
Jason Moore's Twitter - https://twitter.com/jasonmooreme?lang=en
Dr. Jessilyn Dunn's Twitter - https://twitter.com/drjessilyn?lang=en
Dr. Dunn's study on HRV, flu and common cold - https://jamanetwork.com/journals/jamanetworkopen/f...
Dr. Leah Lagos - https://drleahlagos.com/
Dr. Lagos on Star Talk - https://www.youtube.com/watch?v=jC2Q10SonV8
Research on HRV and intermittent fasting - https://pubmed.ncbi.nlm.nih.gov/33859841/
Research on HRV and Mediterranean diet - https://medicalxpress.com/news/2010-06-twin-medite...:~:text=Using%20data%20from%20the%20Emory,eating%20a%20Western%2Dtype%20diet
Devices for HRV biofeedback - https://elitehrv.com/heart-variability-monitors-an...
Benefits of HRV biofeedback - https://pubmed.ncbi.nlm.nih.gov/32385728/
HRV and cognitive performance - https://www.frontiersin.org/articles/10.3389/fnins...
HRV and emotional regulation - https://pubmed.ncbi.nlm.nih.gov/36030986/
Fortune article on HRV - https://fortune.com/well/2022/12/26/heart-rate-var...
Peanut allergies affect about a million children in the U.S., and most never outgrow them. Luckily, some promising remedies are in the works.
Ever since he was a baby, Sharon Wong’s son Brandon suffered from rashes, prolonged respiratory issues and vomiting. In 2006, as a young child, he was diagnosed with a severe peanut allergy.
"My son had a history of reacting to traces of peanuts in the air or in food,” says Wong, a food allergy advocate who runs a blog focusing on nut free recipes, cooking techniques and food allergy awareness. “Any participation in school activities, social events, or travel with his peanut allergy required a lot of preparation.”
Peanut allergies affect around a million children in the U.S. Most never outgrow the condition. The problem occurs when the immune system mistakenly views the proteins in peanuts as a threat and releases chemicals to counteract it. This can lead to digestive problems, hives and shortness of breath. For some, like Wong’s son, even exposure to trace amounts of peanuts could be life threatening. They go into anaphylactic shock and need to take a shot of adrenaline as soon as possible.
Typically, people with peanut allergies try to completely avoid them and carry an adrenaline autoinjector like an EpiPen in case of emergencies. This constant vigilance is very stressful, particularly for parents with young children.
“The search for a peanut allergy ‘cure’ has been a vigorous one,” says Claudia Gray, a pediatrician and allergist at Vincent Pallotti Hospital in Cape Town, South Africa. The closest thing to a solution so far, she says, is the process of desensitization, which exposes the patient to gradually increasing doses of peanut allergen to build up a tolerance. The most common type of desensitization is oral immunotherapy, where patients ingest small quantities of peanut powder. It has been effective but there is a risk of anaphylaxis since it involves swallowing the allergen.
"By the end of the trial, my son tolerated approximately 1.5 peanuts," Sharon Wong says.
DBV Technologies, a company based in Montrouge, France has created a skin patch to address this problem. The Viaskin Patch contains a much lower amount of peanut allergen than oral immunotherapy and delivers it through the skin to slowly increase tolerance. This decreases the risk of anaphylaxis.
Wong heard about the peanut patch and wanted her son to take part in an early phase 2 trial for 4-to-11-year-olds.
“We felt that participating in DBV’s peanut patch trial would give him the best chance at desensitization or at least increase his tolerance from a speck of peanut to a peanut,” Wong says. “The daily routine was quite simple, remove the old patch and then apply a new one. By the end of the trial, he tolerated approximately 1.5 peanuts.”
How it works
For DBV Technologies, it all began when pediatric gastroenterologist Pierre-Henri Benhamou teamed up with fellow professor of gastroenterology Christopher Dupont and his brother, engineer Bertrand Dupont. Together they created a more effective skin patch to detect when babies have allergies to cow's milk. Then they realized that the patch could actually be used to treat allergies by promoting tolerance. They decided to focus on peanut allergies first as the more dangerous.
The Viaskin patch utilizes the fact that the skin can promote tolerance to external stimuli. The skin is the body’s first defense. Controlling the extent of the immune response is crucial for the skin. So it has defense mechanisms against external stimuli and can promote tolerance.
The patch consists of an adhesive foam ring with a plastic film on top. A small amount of peanut protein is placed in the center. The adhesive ring is attached to the back of the patient's body. The peanut protein sits above the skin but does not directly touch it. As the patient sweats, water droplets on the inside of the film dissolve the peanut protein, which is then absorbed into the skin.
The peanut protein is then captured by skin cells called Langerhans cells. They play an important role in getting the immune system to tolerate certain external stimuli. Langerhans cells take the peanut protein to lymph nodes which activate T regulatory cells. T regulatory cells suppress the allergic response.
A different patch is applied to the skin every day to increase tolerance. It’s both easy to use and convenient.
“The DBV approach uses much smaller amounts than oral immunotherapy and works through the skin significantly reducing the risk of allergic reactions,” says Edwin H. Kim, the division chief of Pediatric Allergy and Immunology at the University of North Carolina, U.S., and one of the principal investigators of Viaskin’s clinical trials. “By not going through the mouth, the patch also avoids the taste and texture issues. Finally, the ability to apply a patch and immediately go about your day may be very attractive to very busy patients and families.”
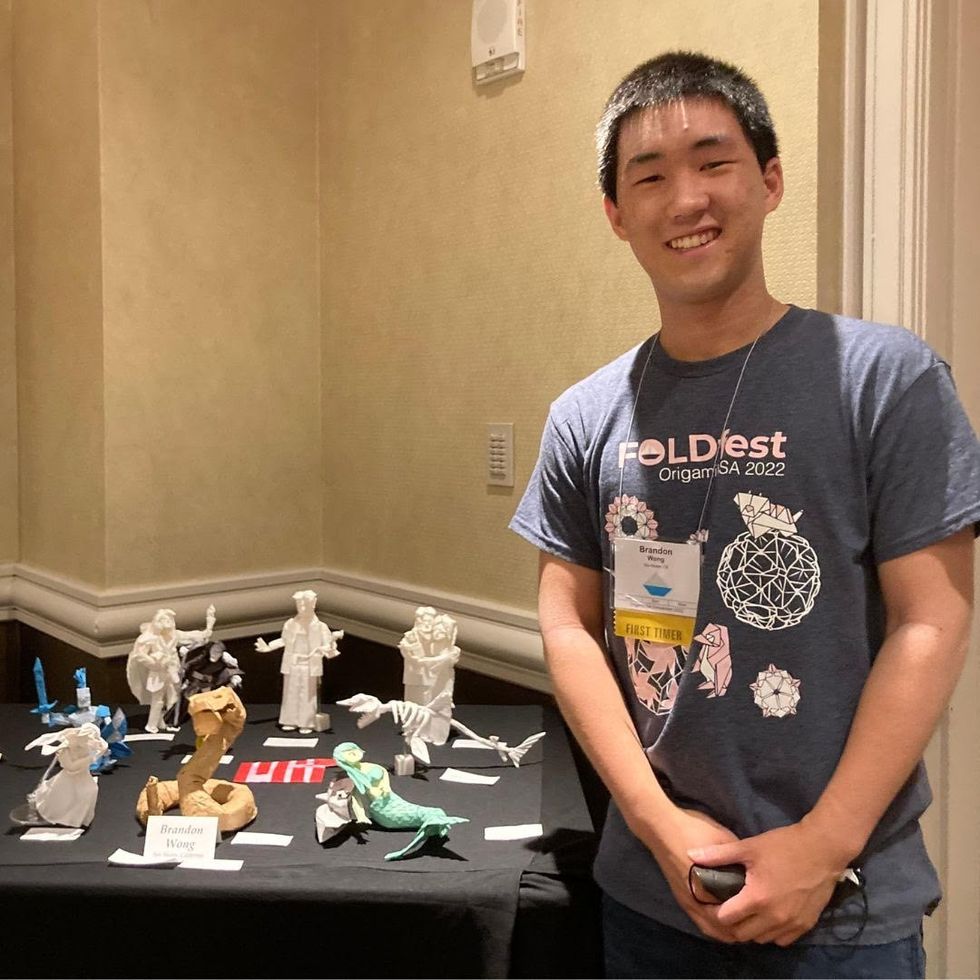
Brandon Wong displaying origami figures he folded at an Origami Convention in 2022
Sharon Wong
Clinical trials
Results from DBV's phase 3 trial in children ages 1 to 3 show its potential. For a positive result, patients who could not tolerate 10 milligrams or less of peanut protein had to be able to manage 300 mg or more after 12 months. Toddlers who could already tolerate more than 10 mg needed to be able to manage 1000 mg or more. In the end, 67 percent of subjects using the Viaskin patch met the target as compared to 33 percent of patients taking the placebo dose.
“The Viaskin peanut patch has been studied in several clinical trials to date with promising results,” says Suzanne M. Barshow, assistant professor of medicine in allergy and asthma research at Stanford University School of Medicine in the U.S. “The data shows that it is safe and well-tolerated. Compared to oral immunotherapy, treatment with the patch results in fewer side effects but appears to be less effective in achieving desensitization.”
The primary reason the patch is less potent is that oral immunotherapy uses a larger amount of the allergen. Additionally, absorption of the peanut protein into the skin could be erratic.
Gray also highlights that there is some tradeoff between risk and efficacy.
“The peanut patch is an exciting advance but not as effective as the oral route,” Gray says. “For those patients who are very sensitive to orally ingested peanut in oral immunotherapy or have an aversion to oral peanut, it has a use. So, essentially, the form of immunotherapy will have to be tailored to each patient.” Having different forms such as the Viaskin patch which is applied to the skin or pills that patients can swallow or dissolve under the tongue is helpful.
The hope is that the patch’s efficacy will increase over time. The team is currently running a follow-up trial, where the same patients continue using the patch.
“It is a very important study to show whether the benefit achieved after 12 months on the patch stays stable or hopefully continues to grow with longer duration,” says Kim, who is an investigator in this follow-up trial.
"My son now attends university in Massachusetts, lives on-campus, and eats dorm food. He has so much more freedom," Wong says.
The team is further ahead in the phase 3 follow-up trial for 4-to-11-year-olds. The initial phase 3 trial was not as successful as the trial for kids between one and three. The patch enabled patients to tolerate more peanuts but there was not a significant enough difference compared to the placebo group to be definitive. The follow-up trial showed greater potency. It suggests that the longer patients are on the patch, the stronger its effects.
They’re also testing if making the patch bigger, changing the shape and extending the minimum time it’s worn can improve its benefits in a trial for a new group of 4-to-11 year-olds.
The future
DBV Technologies is using the skin patch to treat cow’s milk allergies in children ages 1 to 17. They’re currently in phase 2 trials.
As for the peanut allergy trials in toddlers, the hope is to see more efficacy soon.
For Wong’s son who took part in the earlier phase 2 trial for 4-to-11-year-olds, the patch has transformed his life.
“My son continues to maintain his peanut tolerance and is not affected by peanut dust in the air or cross-contact,” Wong says. ”He attends university in Massachusetts, lives on-campus, and eats dorm food. He still carries an EpiPen but has so much more freedom than before his clinical trial. We will always be grateful.”

