Meat Shortages Are Here to Stay. Is Lab-Grown Food a Solution?
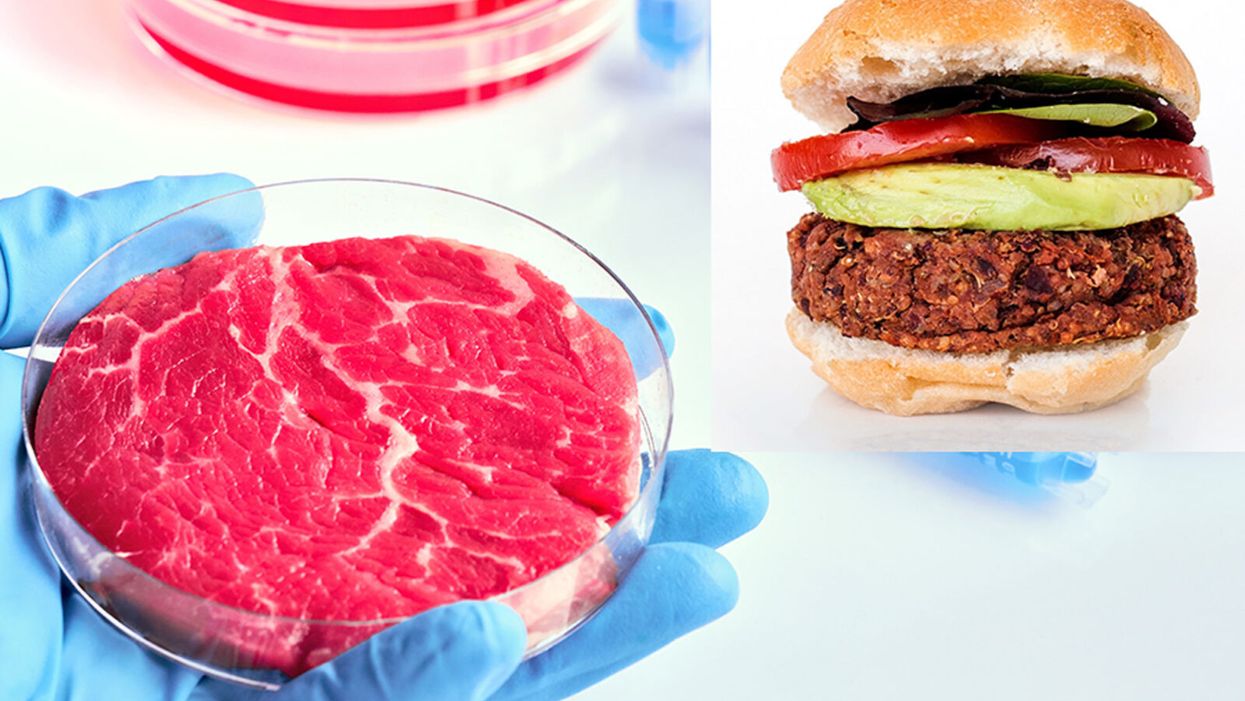
While lab-grown meat is not yet ready to crisis-proof the food supply chain, it offers key benefits that could make it an important solution beyond this pandemic.
The coronavirus pandemic exposed significant weaknesses in the country's food supply chain. Grocery store meat counters were bare. Transportation interruptions influenced supply. Finding beef, poultry, and pork at the store has been, in some places, as challenging as finding toilet paper.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle.
It wasn't a lack of supply -- millions of animals were in the pipeline.
"There's certainly enough food out there, but it can't get anywhere because of the way our system is set up," said Amy Rowat, an associate professor of integrative biology and physiology at UCLA. "Having a more self-contained, self-sufficient way to produce meat could make the supply chain more robust."
Cultured meat could be one way of making the meat supply chain more resilient despite disruptions due to pandemics such as COVID-19. But is the country ready to embrace lab-grown food?
According to a Good Food Institute study, GenZ is almost twice as likely to embrace meat alternatives for reasons related to social and environmental awareness, even prior to the pandemic. That's because this group wants food choices that reflect their values around food justice, equity, and animal welfare.
Largely, the interest in protein alternatives has been plant-based foods. However, factors directly related to COVID-19 may accelerate consumer interest in the scaling up of cell-grown products, according to Liz Specht, the associate director of science and technology at The Good Food Institute. The latter is a nonprofit organization that supports scientists, investors, and entrepreneurs working to develop food alternatives to conventional animal products.
While lab-grown food isn't ready yet to definitively crisis-proof the food supply chain, experts say it offers promise.
Matching Supply and Demand
Companies developing cell-grown meat claim it can take as few as two months to develop a cell into an edible product, according to Anthony Chow, CFA at Agronomics Limited, an investment company focused on meat alternatives. Tissue is taken from an animal and placed in a culture that contains nutrients and proteins the cells need to grow and expand. He cites a Good Food Institute report that claims a 2.5-millimeter sample can grow three and a half tons of meat in 40 days, allowing for exponential growth when needed.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle. To keep enough maturing animals in the pipeline, farms must plan the number of animals to raise months -- even years -- in advance. Lab-grown meat advocates say that because cultured meat supplies can be flexible, it theoretically allows for scaling up or down in significantly less time.
"Supply and demand has drastically changed in some way around the world and cultivated meat processing would be able to adapt much quicker than conventional farming," Chow said.
Scaling Up
Lab-grown meat may provide an eventual solution, but not in the immediate future, said Paul Mozdziak, a professor of physiology at North Carolina State University who researches animal cell culture techniques, transgenic animal production, and muscle biology.
"The challenge is in culture media," he said. "It's going to take some innovation to get the cells to grow at quantities that are going to be similar to what you can get from an animal. These are questions that everybody in the space is working on."
Chow says some of the most advanced cultured meat companies, such as BlueNal, anticipate introducing products to the market midway through next year. However, he thinks COVID-19 has slowed the process. Once introduced, they will be at a premium price, most likely available at restaurants before they hit grocery store shelves.
"I think in five years' time it will be in a different place," he said. "I don't think that this will have relevance for this pandemic, but certainly beyond that."
"Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
Of course, all the technological solutions in the world won't solve the problem unless people are open-minded about embracing them. At least for now, a lab-grown burger or bluefin tuna might still be too strange for many people, especially in the U.S.
For instance, a 2019 article published by "Frontiers in Sustainable Food Systems" reflects results from a study of 3,030 consumers showing that 29 percent of U.S. customers, 59 percent of Chinese consumers, and 56 percent of Indian consumers were either 'very' or 'extremely likely' to try cultivated meat.
"Lab-grown meat is genuine meat, at the cellular level, and therefore will match conventional meat with regard to its nutritional content and overall sensory experience. It could be argued that plant-based meat will never be able to achieve this," says Laura Turner, who works with Chow at Agronomics Limited. "Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
A Solution Beyond This Pandemic
The coronavirus has done more than raise awareness of the fragility of food supply chains. It has also been a wakeup call for consumers and policy makers that it is time to radically rethink our meat, Specht says. Those factors have elevated the profile of lab-grown meat.
"I think the economy is getting a little bit more steam and if I was an investor, I would be getting excited about it," adds Mozdziak.
Beyond crises, Mozdziak explains that as affluence continues to increase globally, meat consumption increases exponentially. Yet farm animals can only grow so quickly and traditional farming won't be able to keep up.
"Even Tyson is saying that by 2050, there's not going to be enough capacity in the animal meat space to meet demand," he notes. "If we don't look at some innovative technologies, how are we going to overcome that?"
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
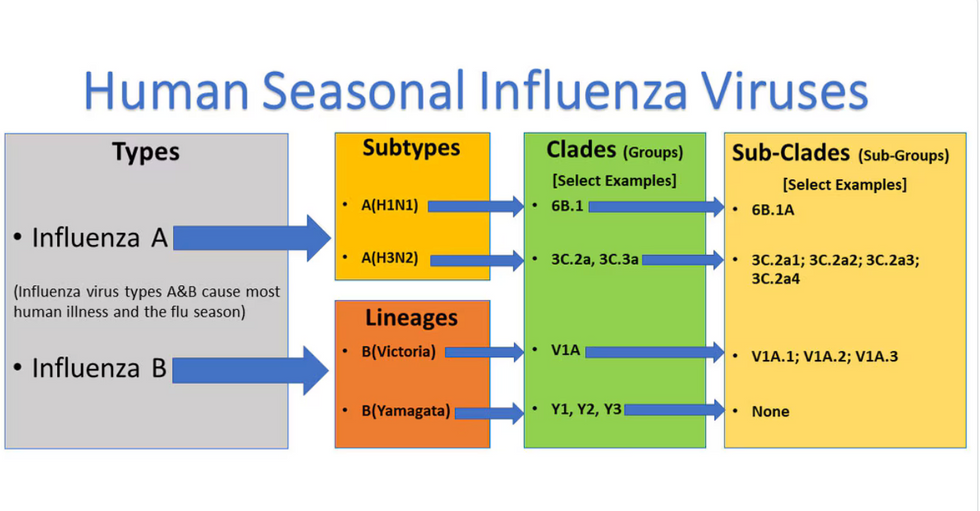
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

