Michio Kaku Talks Life on Mars, Genetic Engineering, and Immortality
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Dr. Michio Kaku
Today is the release of THE FUTURE OF HUMANITY, the latest book by the world-renowned physicist Dr. Michio Kaku. In it, he explores the astonishing technologies that could propel us to live on other planets and even to live forever. LeapsMag Editor-in-Chief Kira Peikoff recently chatted with Dr. Kaku about some of the ethical implications we need to consider as we hurtle toward our destiny among the stars. Our interview has been edited and condensed for clarity.
"Technology is like a double-edged sword. The question is, who wields it?"
A big part of your book discusses living on Mars, and you mention that nanotech, biotech and AI could help us do so in the next 100 years. But you also note that efforts to make the Red Planet habitable could backfire, such as using genetic engineering to produce an ideal fertilizer, which could make one life form push out all the others. How should we judge when a powerful new technology is ready to be tested?
Technology is like a double-edged sword. One side can cut against ignorance, poverty, disease. But the other side can cut against people. The question is, who wields the sword? It has to be wielded by people's interests. We have to look not at the needs of the military or corporations, but society as a whole, and we have to realize that every technology, not just the ones I mentioned in the book, has a dark side as well as a positive side.
On the positive side, you could terraform Mars using genetic engineering to create algae, plants that could thrive in the Martian atmosphere, and a self-sustaining agriculture where we could raise food crops. However, it has to be done carefully, because we don't want to have it overrun Mars, just like we have certain plants that overrun the natural environment here on Earth. So we have to do it slowly. It cannot be done all of a sudden in a crash program. We have to see what happens if we begin to terraform stretches of Martian landscape.
Elon Musk of SpaceX, who has pioneered much of these technologies, has stated that we can jumpstart terraforming Mars by detonating hydrogen bombs over the polar ice caps. Later he had to qualify that by saying that they are airbursts, not ground bursts, to minimize radiation. Other people have said, we don't know what a nuclear weapon would do. Would it destabilize Mars? Would it open cracks in the ice caps? So we have to think things through, not just make proposals. Another proposal is to use silver mirrors in space to reflect sunlight down to melt the ice caps, and that would be more environmentally friendly than using hydrogen bombs.
"Our grandkids, when they hit the age of 30, they may just decide to stop aging, and live at age 30 for many decades to come."
As far as colonizing Mars, you also talk about technologies that could potentially help us end aging, but you note that this could exacerbate overpopulation and an exodus from Earth -- the double-edged sword again. What's your personal view on whether anti-aging research should be pursued?
Anti-aging research is accelerating because of the human genome. We're now able to map the genomes of old people, compare them with the genomes of young people, and we can see where aging takes place. For example, in a car, aging takes place in the engine, because that's where we have moving parts and combustion. Where do we find that in a cell? The mitochondria, and so we do see a concentration of error build-up in the mitochondria, and we can envision one day repairing the mistakes, which could in turn increase our life span. Also we're discovering new enzymes like telomerase which allow us to stop the clock. So it's conceivable, I think not for my generation, but for the coming generations, perhaps our grandkids, when they hit the age of 30, they may just decide to stop aging, and live at age 30 for many decades to come.
The other byproduct of this of course is overpopulation. That's a social problem, but realize in places like Japan, we have the opposite problem, under-population, because the birth rate has fallen way below the replacement level, people live too long, and there's very little immigration there. Europe is next. So we have this bizarre situation where some places like Sub-Saharan Africa are still expanding, but other places we're going to see a contraction. Overall, the population will continue to rise, but it's going to slow down. Instead of this exponential curve that many people see in the media, it's going to be shaped like an "S" that rises rapidly and then seals off. The UN is now beginning to entertain the possibility that the population of the Earth may seal off sometime by the end of the century--that we'll hit a steady state.
"In the future, that composite image may be holographic, with all your videotapes, your memories, to create a near approximation of who you are, and centuries from now, you may have digital immortality."
Later in the book, you talk about achieving immortality through storing your digital consciousness, uploading your brain to a computer. Many people today find that notion bizarre or even repulsive, but you also wisely note that "what seems unethical or even immoral today might be ordinary or mundane in the future." What do you think is the key to bridging the gap between controversial breakthroughs and public acceptance?
I imagine that if someone from the Middle Ages, who is fresh from burning witches and heretics and torturing non-believers, were to wind up today in our society, they might go crazy. They might think all of society is a product of the Devil, because attitudes toward morality change. So we humans today cannot dictate what morality will be like 100 years from now. For example, test tube babies. When Louise Brown (the first test tube baby) was first born, the Catholic Church denounced it. Now, today, your wife, husband, you may be a test tube baby and we don't even blink.
There's a Silicon Valley company today that will take what is known about you on the Internet, your credit card transactions, your emails, and create a composite image of you. In the future, that composite image may be holographic, with all your videotapes, your memories, to create a near approximation of who you are, and centuries from now, you may have digital immortality—your memories, your sensations, will be recorded accurately, and an avatar will recreate it. Like for example, I wouldn't mind talking to Einstein. I wouldn't mind sitting down with the guy and having a great conversation about the universe.
And the Connectome Project, by the end of the century, will map the entire brain--that's every neuron--just like the genome project has mapped every gene. And we live with it, we don't even think twice about the fact that our genome exists. In the future, our connectome will also exist. And the connectome can reproduce your thoughts, your dreams, your sensations. We'll just live with that fact; it will be considered ordinary.
"A hundred years from now, we may want to merge with some of these technologies, rather than have to compete with robots."
Wow. In such a "post-human" era, our bodies could be replaced by robots or maintained by genetic engineering. Once these technologies become commercially available, do you think people should have the freedom to make changes or enhancements to themselves?
I think there should be laws passed at a certain point to prevent parents from going crazy trying to genetically engineer their child. Once we isolate the genes for studying, for good behavior, things like that, we may be tempted to tinker with it. I think a certain amount of tinkering is fine, but we don't want to let it get out of control. There has to be limits.
Also, we are in competition with robots of the future. A hundred years from now, robots are going to become very intelligent. Some people think they're going to take over. My attitude is that a hundred years from now, we may want to merge with some of these technologies, rather than have to compete with robots. But we're not going to look like some freaky robot because we're genetically hardwired to look good to the opposite sex, to look good to our peers. Hundreds of thousands of years ago, and hundreds of thousands of years into the future, we'll still look the same. We'll genetically modify ourselves a little bit, but we'll basically look the same.
That's an interesting point. It's amazing how fast technology is moving overall. Like at one point in the book, you mention that primates had never been cloned, but a few weeks ago, news broke that this just happened in China. Do you think we should slow down the dramatic pace of acceleration and focus on the ethical considerations, or should we still move full-steam ahead?
Well, CRISPR technology has accelerated us more than we previously thought. In the past, to tinker with genes, you had to cut and splice, and it was a lot of guesswork and trial and error. Now, you can zero in on the cutting process and streamline it, so cutting and splicing genes becomes much more accurate, and you can edit them just like you edit a book. Within the field of bioengineering, they have set up their own conferences to begin to police themselves into figuring out which domains are ethically dangerous and which areas can provide benefits for humanity, because they realize that this technology can go a little bit too fast.
"Where does truth come from? Truth comes from interaction with incorrect ideas."
You cannot recall a life form. Once a life form is created, it reproduces. That's what life does. If it reproduces outside the laboratory, it could take over. So we want to make sure that we don't have to recall a life form, like you would recall a Ford or a Chevrolet. Eventually governments may have to slow down the pace because it's moving very rapidly.
Lastly, you talk about the importance of democratic debate to resolve how controversial technology should be used. How can science-minded people bring the rest of society into these conversations, so that as much of society as possible is represented?
It's a question of where does truth come from? Truth comes from interaction with incorrect ideas--the collision of truth and untruth, rumors and fact. It doesn't come from a machine where you put in a quarter, and out comes the answer. It requires democratic debate. And that's where the Internet comes in, that's where the media comes in, that's where this interview comes in. You want to stimulate and educate the people so they know the dangers and promises of technology, and then engage with them about the moral implications, because these things are going to affect every aspect of our life in the future.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
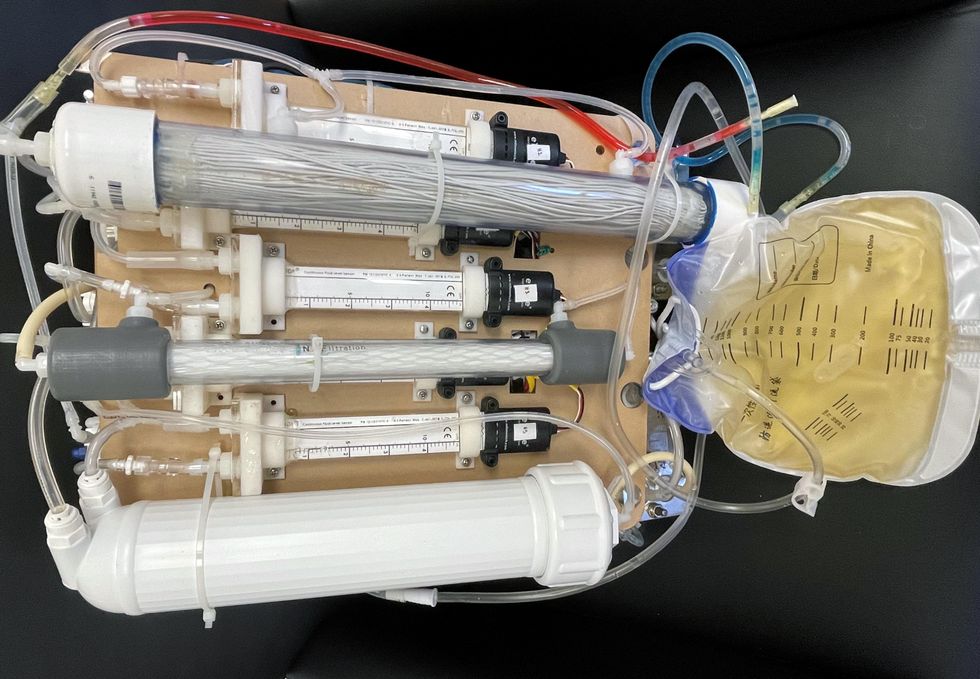
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
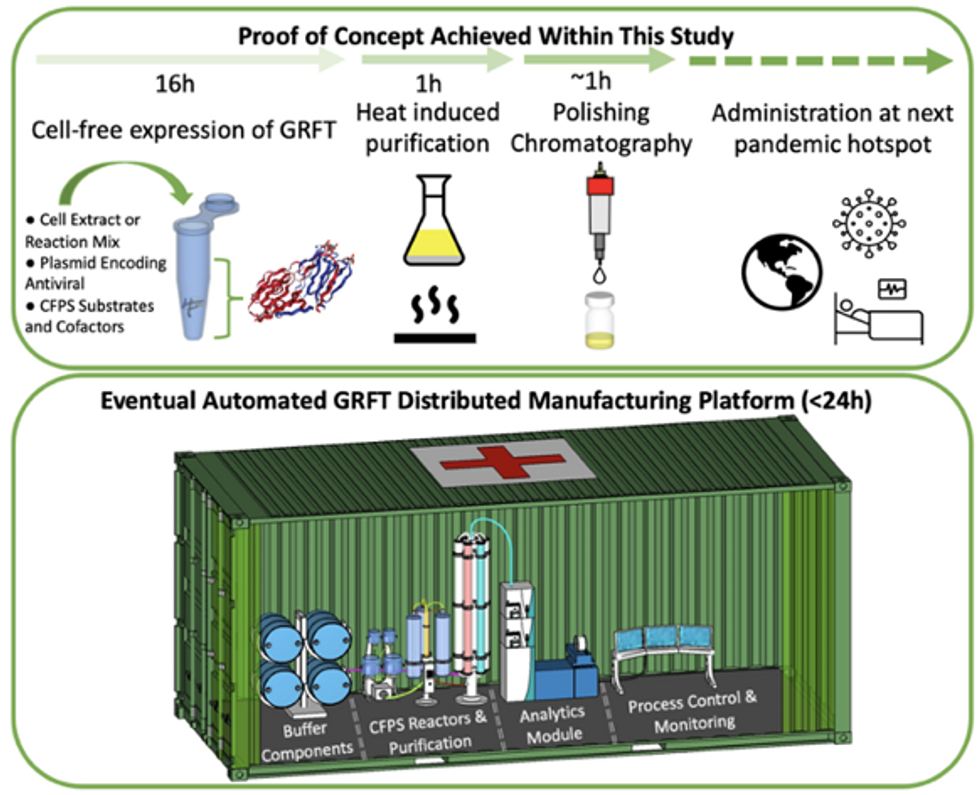
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.