Should We Use Technologies to Enhance Morality?

Should we welcome biomedical technologies that could enhance our ability to tell right from wrong and improve behaviors that are considered immoral such as dishonesty, prejudice and antisocial aggression?
Our moral ‘hardware’ evolved over 100,000 years ago while humans were still scratching the savannah. The perils we encountered back then were radically different from those that confront us now. To survive and flourish in the face of complex future challenges our archaic operating systems might need an upgrade – in non-traditional ways.
Morality refers to standards of right and wrong when it comes to our beliefs, behaviors, and intentions. Broadly, moral enhancement is the use of biomedical technology to improve moral functioning. This could include augmenting empathy, altruism, or moral reasoning, or curbing antisocial traits like outgroup bias and aggression.
The claims related to moral enhancement are grand and polarizing: it’s been both tendered as a solution to humanity’s existential crises and bluntly dismissed as an armchair hypothesis. So, does the concept have any purchase? The answer leans heavily on our definition and expectations.
One issue is that the debate is often carved up in dichotomies – is moral enhancement feasible or unfeasible? Permissible or impermissible? Fact or fiction? On it goes. While these gesture at imperatives, trading in absolutes blurs the realities at hand. A sensible approach must resist extremes and recognize that moral disrupters are already here.
We know that existing interventions, whether they occur unknowingly or on purpose, have the power to modify moral dispositions in ways both good and bad. For instance, neurotoxins can promote antisocial behavior. The ‘lead-crime hypothesis’ links childhood lead-exposure to impulsivity, antisocial aggression, and various other problems. Mercury has been associated with cognitive deficits, which might impair moral reasoning and judgement. It’s well documented that alcohol makes people more prone to violence.
So, what about positive drivers? Here’s where it gets more tangled.
Medicine has long treated psychiatric disorders with drugs like sedatives and antipsychotics. However, there’s short mention of morality in the Diagnostic and Statistical Manual of Mental Disorders (DSM) despite the moral merits of pharmacotherapy – these effects are implicit and indirect. Such cases are regarded as treatments rather than enhancements.
It would be dangerously myopic to assume that moral augmentation is somehow beyond reach.
Conventionally, an enhancement must go beyond what is ‘normal,’ species-typical, or medically necessary – this is known as the ‘treatment-enhancement distinction.’ But boundaries of health and disease are fluid, so whether we call a procedure ‘moral enhancement’ or ‘medical treatment’ is liable to change with shifts in social values, expert opinions, and clinical practices.
Human enhancements are already used for a range of purported benefits: caffeine, smart drugs, and other supplements to boost cognitive performance; cosmetic procedures for aesthetic reasons; and steroids and stimulants for physical advantage. More boldly, cyborgs like Moon Ribas and Neil Harbisson are pushing transpecies boundaries with new kinds of sensory perception. It would be dangerously myopic to assume that moral augmentation is somehow beyond reach.
How might it work?
One possibility for shaping moral temperaments is with neurostimulation devices. These use electrodes to deliver a low-intensity current that alters the electromagnetic activity of specific neural regions. For instance, transcranial Direct Current Stimulation (tDCS) can target parts of the brain involved in self-awareness, moral judgement, and emotional decision-making. It’s been shown to increase empathy and valued-based learning, and decrease aggression and risk-taking behavior. Many countries already use tDCS to treat pain and depression, but evidence for enhancement effects on healthy subjects is mixed.
Another suggestion is targeting neuromodulators like serotonin and dopamine. Serotonin is linked to prosocial attributes like trust, fairness, and cooperation, but low activity is thought to motivate desires for revenge and harming others. It’s not as simple as indiscriminately boosting brain chemicals though. While serotonin is amenable to SSRIs, precise levels are difficult to measure and track, and there’s no scientific consensus on the “optimum” amount or on whether such a value even exists. Fluctuations due to lifestyle factors such as diet, stress, and exercise add further complexity. Currently, more research is needed on the significance of neuromodulators and their network dynamics across the moral landscape.
There are a range of other prospects. The ‘love drugs’ oxytocin and MDMA mediate pair bonding, cooperation, and social attachment, although some studies suggest that people with high levels of oxytocin are more aggressive toward outsiders. Lithium is a mood stabilizer that has been shown to reduce aggression in prison populations; beta-blockers like propranolol and the supplement omega-3 have similar effects. Increasingly, brain-computer interfaces augur a world of brave possibilities. Such appeals are not without limitations, but they indicate some ways that external tools can positively nudge our moral sentiments.
Who needs morally enhancing?
A common worry is that enhancement technologies could be weaponized for social control by authoritarian regimes, or used like the oppressive eugenics of the early 20th century. Fortunately, the realities are far more mundane and such dystopian visions are fantastical. So, what are some actual possibilities?
Some researchers suggest that neurotechnologies could help to reactivate brain regions of those suffering from moral pathologies, including healthy people with psychopathic traits (like a lack of empathy). Another proposal is using such technology on young people with conduct problems to prevent serious disorders in adulthood.
Most of us aren’t always as ethical as we would like – given the option of ‘priming’ yourself to act in consistent accord with your higher values, would you take it?
A question is whether these kinds of interventions should be compulsory for dangerous criminals. On the other hand, a voluntary treatment for inmates wouldn’t be so different from existing incentive schemes. For instance, some U.S. jurisdictions already offer drug treatment programs in exchange for early release or instead of prison time. Then there’s the difficult question of how we should treat non-criminal but potentially harmful ‘successful’ psychopaths.
Others argue that if virtues have a genetic component, there is no technological reason why present practices of embryo screening for genetic diseases couldn’t also be used for selecting socially beneficial traits.
Perhaps the most immediate scenario is a kind of voluntary moral therapy, which would use biomedicine to facilitate ideal brain-states to augment traditional psychotherapy. Most of us aren’t always as ethical as we would like – given the option of ‘priming’ yourself to act in consistent accord with your higher values, would you take it? Approaches like neurofeedback and psychedelic-assisted therapy could prove helpful.
What are the challenges?
A general challenge is that of setting. Morality is context dependent; what’s good in one environment may be bad in another and vice versa, so we don’t want to throw out the baby with the bathwater. Of course, common sense tells us that some tendencies are more socially desirable than others: fairness, altruism, and openness are clearly preferred over aggression, dishonesty, and prejudice.
One argument is that remoulding ‘brute impulses’ via biology would not count as moral enhancement. This view claims that for an action to truly count as moral it must involve cognition – reasoning, deliberation, judgement – as a necessary part of moral behavior. Critics argue that we should be concerned more with ends rather than means, so ultimately it’s outcomes that matter most.
Another worry is that modifying one biological aspect will have adverse knock-on effects for other valuable traits. Certainly, we must be careful about the network impacts of any intervention. But all stimuli have distributed effects on the body, so it’s really a matter of weighing up the cost/benefit trade-offs as in any standard medical decision.
Is it ethical?
Our values form a big part of who we are – some bioethicists argue that altering morality would pose a threat to character and personal identity. Another claim is that moral enhancement would compromise autonomy by limiting a person’s range of choices and curbing their ‘freedom to fall.’ Any intervention must consider the potential impacts on selfhood and personal liberty, in addition to the wider social implications.
This includes the importance of social and genetic diversity, which is closely tied to considerations of fairness, equality, and opportunity. The history of psychiatry is rife with examples of systematic oppression, like ‘drapetomania’ – the spurious mental illness that was thought to cause African slaves’ desire to flee captivity. Advocates for using moral enhancement technologies to help kids with conduct problems should be mindful that they disproportionately come from low-income communities. We must ensure that any habilitative practice doesn’t perpetuate harmful prejudices by unfairly targeting marginalized people.
Human capacities are the result of environmental influences, and external conditions still coax our biology in unknown ways. Status quo bias for ‘letting nature take its course’ may actually be worse long term – failing to utilize technology for human development may do more harm than good.
Then, there are concerns that morally-enhanced persons would be vulnerable to predation by those who deliberately avoid moral therapies. This relates to what’s been dubbed the ‘bootstrapping problem’: would-be moral enhancement candidates are the types of individuals that benefit from not being morally enhanced. Imagine if every senator was asked to undergo an honesty-boosting procedure prior to entering public office – would they go willingly? Then again, perhaps a technological truth-serum wouldn’t be such a bad requisite for those in positions of stern social consequence.
Advocates argue that biomedical moral betterment would simply offer another means of pursuing the same goals as fixed social mechanisms like religion, education, and community, and non-invasive therapies like cognitive-behavior therapy and meditation. It’s even possible that technological efforts would be more effective. After all, human capacities are the result of environmental influences, and external conditions still coax our biology in unknown ways. Status quo bias for ‘letting nature take its course’ may actually be worse long term – failing to utilize technology for human development may do more harm than good. If we can safely improve ourselves in direct and deliberate ways then there’s no morally significant difference whether this happens via conventional methods or new technology.
Future prospects
Where speculation about human enhancement has led to hype and technophilia, many bioethicists urge restraint. We can be grounded in current science while anticipating feasible medium-term prospects. It’s unlikely moral enhancement heralds any metamorphic post-human utopia (or dystopia), but that doesn’t mean dismissing its transformative potential. In one sense, we should be wary of transhumanist fervour about the salvatory promise of new technology. By the same token we must resist technofear and alarmist efforts to balk social and scientific progress. Emerging methods will continue to shape morality in subtle and not-so-subtle ways – the critical steps are spotting and scaffolding these with robust ethical discussion, public engagement, and reasonable policy options. Steering a bright and judicious course requires that we pilot the possibilities of morally-disruptive technologies.
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
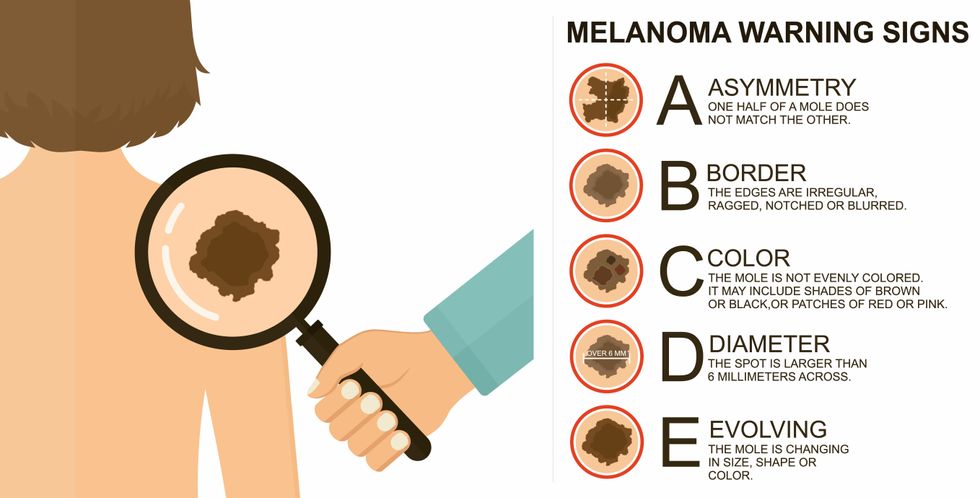
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

