Should We Use Technologies to Enhance Morality?

Should we welcome biomedical technologies that could enhance our ability to tell right from wrong and improve behaviors that are considered immoral such as dishonesty, prejudice and antisocial aggression?
Our moral ‘hardware’ evolved over 100,000 years ago while humans were still scratching the savannah. The perils we encountered back then were radically different from those that confront us now. To survive and flourish in the face of complex future challenges our archaic operating systems might need an upgrade – in non-traditional ways.
Morality refers to standards of right and wrong when it comes to our beliefs, behaviors, and intentions. Broadly, moral enhancement is the use of biomedical technology to improve moral functioning. This could include augmenting empathy, altruism, or moral reasoning, or curbing antisocial traits like outgroup bias and aggression.
The claims related to moral enhancement are grand and polarizing: it’s been both tendered as a solution to humanity’s existential crises and bluntly dismissed as an armchair hypothesis. So, does the concept have any purchase? The answer leans heavily on our definition and expectations.
One issue is that the debate is often carved up in dichotomies – is moral enhancement feasible or unfeasible? Permissible or impermissible? Fact or fiction? On it goes. While these gesture at imperatives, trading in absolutes blurs the realities at hand. A sensible approach must resist extremes and recognize that moral disrupters are already here.
We know that existing interventions, whether they occur unknowingly or on purpose, have the power to modify moral dispositions in ways both good and bad. For instance, neurotoxins can promote antisocial behavior. The ‘lead-crime hypothesis’ links childhood lead-exposure to impulsivity, antisocial aggression, and various other problems. Mercury has been associated with cognitive deficits, which might impair moral reasoning and judgement. It’s well documented that alcohol makes people more prone to violence.
So, what about positive drivers? Here’s where it gets more tangled.
Medicine has long treated psychiatric disorders with drugs like sedatives and antipsychotics. However, there’s short mention of morality in the Diagnostic and Statistical Manual of Mental Disorders (DSM) despite the moral merits of pharmacotherapy – these effects are implicit and indirect. Such cases are regarded as treatments rather than enhancements.
It would be dangerously myopic to assume that moral augmentation is somehow beyond reach.
Conventionally, an enhancement must go beyond what is ‘normal,’ species-typical, or medically necessary – this is known as the ‘treatment-enhancement distinction.’ But boundaries of health and disease are fluid, so whether we call a procedure ‘moral enhancement’ or ‘medical treatment’ is liable to change with shifts in social values, expert opinions, and clinical practices.
Human enhancements are already used for a range of purported benefits: caffeine, smart drugs, and other supplements to boost cognitive performance; cosmetic procedures for aesthetic reasons; and steroids and stimulants for physical advantage. More boldly, cyborgs like Moon Ribas and Neil Harbisson are pushing transpecies boundaries with new kinds of sensory perception. It would be dangerously myopic to assume that moral augmentation is somehow beyond reach.
How might it work?
One possibility for shaping moral temperaments is with neurostimulation devices. These use electrodes to deliver a low-intensity current that alters the electromagnetic activity of specific neural regions. For instance, transcranial Direct Current Stimulation (tDCS) can target parts of the brain involved in self-awareness, moral judgement, and emotional decision-making. It’s been shown to increase empathy and valued-based learning, and decrease aggression and risk-taking behavior. Many countries already use tDCS to treat pain and depression, but evidence for enhancement effects on healthy subjects is mixed.
Another suggestion is targeting neuromodulators like serotonin and dopamine. Serotonin is linked to prosocial attributes like trust, fairness, and cooperation, but low activity is thought to motivate desires for revenge and harming others. It’s not as simple as indiscriminately boosting brain chemicals though. While serotonin is amenable to SSRIs, precise levels are difficult to measure and track, and there’s no scientific consensus on the “optimum” amount or on whether such a value even exists. Fluctuations due to lifestyle factors such as diet, stress, and exercise add further complexity. Currently, more research is needed on the significance of neuromodulators and their network dynamics across the moral landscape.
There are a range of other prospects. The ‘love drugs’ oxytocin and MDMA mediate pair bonding, cooperation, and social attachment, although some studies suggest that people with high levels of oxytocin are more aggressive toward outsiders. Lithium is a mood stabilizer that has been shown to reduce aggression in prison populations; beta-blockers like propranolol and the supplement omega-3 have similar effects. Increasingly, brain-computer interfaces augur a world of brave possibilities. Such appeals are not without limitations, but they indicate some ways that external tools can positively nudge our moral sentiments.
Who needs morally enhancing?
A common worry is that enhancement technologies could be weaponized for social control by authoritarian regimes, or used like the oppressive eugenics of the early 20th century. Fortunately, the realities are far more mundane and such dystopian visions are fantastical. So, what are some actual possibilities?
Some researchers suggest that neurotechnologies could help to reactivate brain regions of those suffering from moral pathologies, including healthy people with psychopathic traits (like a lack of empathy). Another proposal is using such technology on young people with conduct problems to prevent serious disorders in adulthood.
Most of us aren’t always as ethical as we would like – given the option of ‘priming’ yourself to act in consistent accord with your higher values, would you take it?
A question is whether these kinds of interventions should be compulsory for dangerous criminals. On the other hand, a voluntary treatment for inmates wouldn’t be so different from existing incentive schemes. For instance, some U.S. jurisdictions already offer drug treatment programs in exchange for early release or instead of prison time. Then there’s the difficult question of how we should treat non-criminal but potentially harmful ‘successful’ psychopaths.
Others argue that if virtues have a genetic component, there is no technological reason why present practices of embryo screening for genetic diseases couldn’t also be used for selecting socially beneficial traits.
Perhaps the most immediate scenario is a kind of voluntary moral therapy, which would use biomedicine to facilitate ideal brain-states to augment traditional psychotherapy. Most of us aren’t always as ethical as we would like – given the option of ‘priming’ yourself to act in consistent accord with your higher values, would you take it? Approaches like neurofeedback and psychedelic-assisted therapy could prove helpful.
What are the challenges?
A general challenge is that of setting. Morality is context dependent; what’s good in one environment may be bad in another and vice versa, so we don’t want to throw out the baby with the bathwater. Of course, common sense tells us that some tendencies are more socially desirable than others: fairness, altruism, and openness are clearly preferred over aggression, dishonesty, and prejudice.
One argument is that remoulding ‘brute impulses’ via biology would not count as moral enhancement. This view claims that for an action to truly count as moral it must involve cognition – reasoning, deliberation, judgement – as a necessary part of moral behavior. Critics argue that we should be concerned more with ends rather than means, so ultimately it’s outcomes that matter most.
Another worry is that modifying one biological aspect will have adverse knock-on effects for other valuable traits. Certainly, we must be careful about the network impacts of any intervention. But all stimuli have distributed effects on the body, so it’s really a matter of weighing up the cost/benefit trade-offs as in any standard medical decision.
Is it ethical?
Our values form a big part of who we are – some bioethicists argue that altering morality would pose a threat to character and personal identity. Another claim is that moral enhancement would compromise autonomy by limiting a person’s range of choices and curbing their ‘freedom to fall.’ Any intervention must consider the potential impacts on selfhood and personal liberty, in addition to the wider social implications.
This includes the importance of social and genetic diversity, which is closely tied to considerations of fairness, equality, and opportunity. The history of psychiatry is rife with examples of systematic oppression, like ‘drapetomania’ – the spurious mental illness that was thought to cause African slaves’ desire to flee captivity. Advocates for using moral enhancement technologies to help kids with conduct problems should be mindful that they disproportionately come from low-income communities. We must ensure that any habilitative practice doesn’t perpetuate harmful prejudices by unfairly targeting marginalized people.
Human capacities are the result of environmental influences, and external conditions still coax our biology in unknown ways. Status quo bias for ‘letting nature take its course’ may actually be worse long term – failing to utilize technology for human development may do more harm than good.
Then, there are concerns that morally-enhanced persons would be vulnerable to predation by those who deliberately avoid moral therapies. This relates to what’s been dubbed the ‘bootstrapping problem’: would-be moral enhancement candidates are the types of individuals that benefit from not being morally enhanced. Imagine if every senator was asked to undergo an honesty-boosting procedure prior to entering public office – would they go willingly? Then again, perhaps a technological truth-serum wouldn’t be such a bad requisite for those in positions of stern social consequence.
Advocates argue that biomedical moral betterment would simply offer another means of pursuing the same goals as fixed social mechanisms like religion, education, and community, and non-invasive therapies like cognitive-behavior therapy and meditation. It’s even possible that technological efforts would be more effective. After all, human capacities are the result of environmental influences, and external conditions still coax our biology in unknown ways. Status quo bias for ‘letting nature take its course’ may actually be worse long term – failing to utilize technology for human development may do more harm than good. If we can safely improve ourselves in direct and deliberate ways then there’s no morally significant difference whether this happens via conventional methods or new technology.
Future prospects
Where speculation about human enhancement has led to hype and technophilia, many bioethicists urge restraint. We can be grounded in current science while anticipating feasible medium-term prospects. It’s unlikely moral enhancement heralds any metamorphic post-human utopia (or dystopia), but that doesn’t mean dismissing its transformative potential. In one sense, we should be wary of transhumanist fervour about the salvatory promise of new technology. By the same token we must resist technofear and alarmist efforts to balk social and scientific progress. Emerging methods will continue to shape morality in subtle and not-so-subtle ways – the critical steps are spotting and scaffolding these with robust ethical discussion, public engagement, and reasonable policy options. Steering a bright and judicious course requires that we pilot the possibilities of morally-disruptive technologies.
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
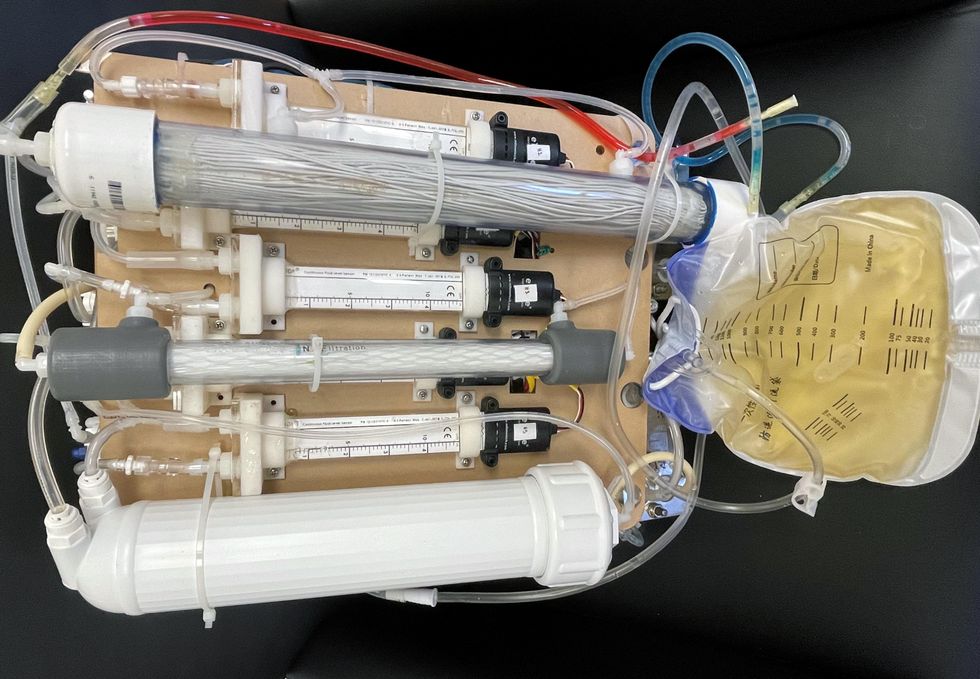
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
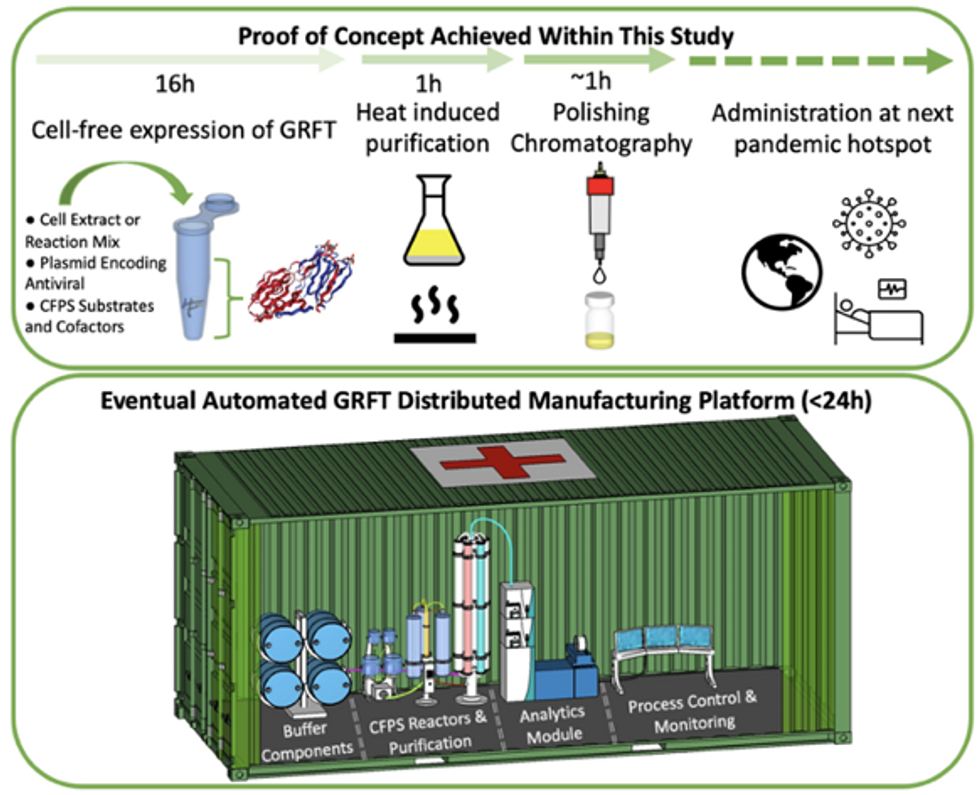
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.