CRISPR base editing gives measure of hope to people with muscular dystrophy

Next year, a neurologist will test CRISPR base editing in a trial of five people with muscular dystrophy to see if their muscles accept corrected cells and whether they multiply and take over the function of damaged cells.
When Martin Weber climbs the steps to his apartment on the fifth floor in Munich, an attentive observer might notice that he walks a little unevenly. “That’s because my calf muscles were the first to lose strength,” Weber explains.
About three years ago, the now 19-year-old university student realized that he suddenly had trouble keeping up with his track team at school. At tennis tournaments, he seemed to lose stamina after the first hour. “But it was still within the norm,” he says. “So it took a while before I noticed something was seriously wrong.” A blood test showed highly elevated liver markers. His parents feared he had liver cancer until a week-long hospital visit and scores of tests led to a diagnosis: hereditary limb-girdle muscular dystrophy, an incurable genetic illness that causes muscles to deteriorate.
As you read this text, you will surely use several muscles without being aware of them: Your heart muscle pumps blood through your arteries, your eye muscles let you follow the words in this sentence, and your hand muscles hold the tablet or cell phone. Muscles make up 40 percent of your body weight; we usually have 656 of them. Now imagine they are slowly losing their strength. No training, no protein shake can rebuild their function.
This is the reality for most people in Simone Spuler’s outpatient clinic at the Charité Hospital in Berlin, Germany: Almost all of her 2,500 patients have muscular dystrophy, a progressive illness striking mostly young people. Muscle decline leads to a wheelchair and, eventually, an early death due to a heart attack or the inability to breathe. In Germany alone, 300,000 people live with this illness, the youngest barely a year old. The CDC estimates that its most common form, Duchenne, affects 1 in every 3,500 to 6,000 male births each year in the United States.
The devastating progression of the disease is what motivates Spuler and her team of 25 scientists to find a cure. In 2019, they made a spectacular breakthrough: For the first time, they successfully used mRNA to introduce the CRISPR-Cas9 tool into human muscle stem cells to repair the dystrophy. “It’s really just one tiny molecule that doesn’t work properly,” Spuler explains.
CRISPR-Cas9 is a technology that lets scientists select and alter parts of the genome. It’s still comparatively new but has advanced quickly since its discovery in the early 2010s. “We now have the possibility to repair certain mutations with genetic editing,” Spuler says. “It’s pure magic.”
She projects a warm, motherly air and a professional calm that inspires trust from her patients. She needs these qualities because the 60-year-old neurologist has one of the toughest jobs in the world: All day long, patients with the incurable diagnosis of muscular dystrophy come to her clinic, and she watches them decline over the years. “Apart from physiotherapy, there is nothing we can recommend right now,” she says. That motivated her early in her career, when she met her first patients at the Max Planck Institute for Neurobiology near Munich in the 1990s. “I knew I had 30, 40 years to find something.”
She learned from the luminaries of her profession with postdocs at the University of California San Diego, Harvard and Johns Hopkins, before serving as a clinical fellow at the Mayo Clinic. In 2005, the Charité offered her the opportunity to establish a specialized clinic for myasthenia, or muscular weakness. An important influence on Spuler, she says, has been the French microbiologist Emmanuelle Charpentier, who received the Nobel Prize in 2020 along with Jennifer Doudna for their CRISPR research, and has worked in Berlin since 2015.
When CRISPR was first introduced, it was mainly used to cut through DNA. However, the cut can lead to undesired side effects. For the muscle stem cells, Spuler now uses a base editor to repair the damaged molecule with super fine scissors or tweezers.

“Apart from physiotherapy, there is nothing we can recommend right now,” Spuler says about her patients with limb-girdle muscular dystrophy.
Pablo Castagnola
Last year, she proved that the method works in mice. Injecting repaired cells into the rodents led to new muscle fibers and, in 2021 and 2022, she passed the first safety meetings with the Paul-Ehrlich Institute, which is responsible for approving human gene editing trials in Germany. She raised the nearly four million Euros needed to test the new method in the first clinical trial in humans with limb-girdle muscular dystrophy, beginning with one muscle that can easily be measured, such as the biceps.
This spring, Weber and his parents drove the 400 miles from Munich to Berlin. At Spuler’s lab, her team took a biopsy from muscles in his left arm. The first two steps – extraction and repair in a culture dish – went according to plan; Spuler was able to repair the mutation in Weber’s cells outside his body.
Next year, Weber will be the youngest participant when Spuler starts to test the method in a trial of five people “in vivo,” inside their bodies. This will be the real moment of truth: Will the participants’ muscles accept the corrected cells? Will the cells multiply and take over the function of damaged cells, just like Spuler was able to do in her lab with the rodents?
The effort is costly and complex. “The biggest challenge is to make absolutely sure that we don’t harm the patient,” Spuler says. This means scanning their entire genomes, “so we don’t accidentally damage or knock out an important gene.”
Weber, who asked not to be identified by his real name, is looking forward to the trial and he feels confident that “the risks are comparatively small because the method will only be applied to one muscle. The worst that can happen is that it doesn’t work. But in the best case, the muscle function will improve.”
He was so impressed with the Charité scientists that he decided to study biology at his university. He’s read extensively about CRISPR, so he understands why he has three healthy siblings. “That’s the statistics,” the biologist in training explains. “You get two sets of genes from each parent, and you have to get two faulty mutations to have muscular dystrophy. So we fit the statistics exactly: One of us four kids inherited the mutation.”
It was his mother, a college teacher, and father, a physicist by training, who heard about Spuler’s research. Even though Weber does not live at home anymore, having a chronically ill son is nearly a full-time job for his mother, Annette. The Berlin visit and the trial are financed separately through private sponsors, but the fights with Weber’s health insurance are frustrating and time-consuming. “Physiotherapy is the only thing that helps a bit,” Weber says, “and yet, they fought us on approving it every step of the way.”
Spuler does not want to evoke unrealistic expectations. “Patients who are wheelchair-bound won’t suddenly get up and walk."
Her son continues to exercise as much as possible. Riding his bicycle to the university has become too difficult, so he got an e-scooter. He had to give up competitive tennis because he does not have the stamina for a two-hour match, but he can still play with his dad or his buddies for an hour. His closest friends know about the diagnosis. “They help me, for instance, to lift something heavy because I can’t do that anymore,” Weber says.
The family was elated to find medical support at the Munich Muscle Center by the German Alliance for Muscular Patients and then at Spuler’s clinic in Berlin. “When you hear that this is a progressive illness with no chance of improvement, your world collapses as a parent,” Annette Weber says. “And then all of a sudden, there is this woman who sees scientific progress as an opportunity. Even just to be able to participate in the study is fantastic.”
Spuler does not want to evoke unrealistic expectations. “Patients who are wheelchair-bound won’t suddenly get up and walk,” she says. After all, she will start by applying the gene editor to only one muscle, “but it would be a big step if even a small muscle that is essential to grip something, or to swallow, regains function.”
Weber agrees. “I understand that I won’t regain 100 percent of my muscle function but even a small improvement or at least halting the deterioration is the goal.”
And yet, Spuler and others are ultimately searching for a true solution. In a separate effort, Massachusetts-based biotech company Sarepta announced this month it will seek expedited regulators’ approval to treat Duchenne patients with its investigational gene therapy. Unlike Spuler’s methods, Sarepta focuses specifically on the Duchenne form of muscular dystrophy, and it uses an adeno-assisted virus to deliver the therapy.
Spuler’s vision is to eventually apply gene editing to the entire body of her patients. To speed up the research, she and a colleague founded a private research company, Myopax. If she is able to prove that the body accepts the edited cells, the technique could be used for other monogenetic illnesses as well. “When we speak of genetic editing, many are scared and say, oh no, this is God’s work,” says Spuler. But she sees herself as a mechanic, not a divine being. “We really just exchange a molecule, that’s it.”
If everything goes well, Weber hopes that ten years from now, he will be the one taking biopsies from the next generation of patients and repairing their genes.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
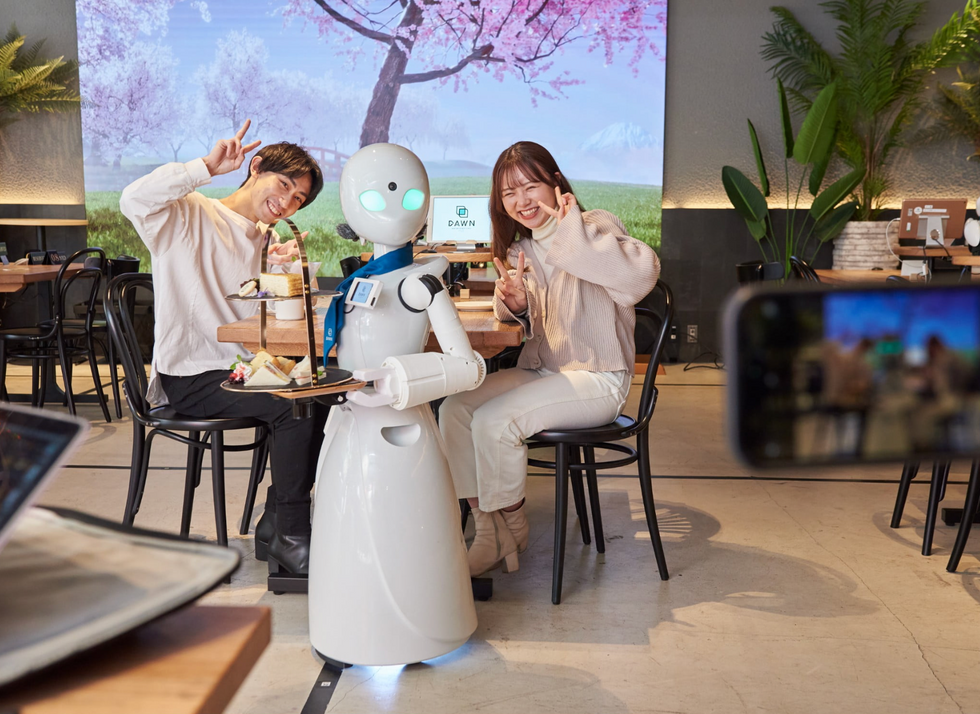
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

