Why Neglected Tropical Diseases Should Matter to Americans

Kissing bugs can carry a parasite called Trypanosoma cruzi, which causes Chagas disease.
Daisy Hernández was five years old when one of her favorite aunts was struck with a mysterious illness. Tía Dora had stayed behind in Colombia when Daisy's mother immigrated to Union City, New Jersey. A schoolteacher in her late 20s, she began suffering from fevers and abdominal pain, and her belly grew so big that people thought she was pregnant. Exploratory surgery revealed that her large intestine had swollen to ten times its normal size, and she was fitted with a colostomy bag. Doctors couldn't identify the underlying problem—but whatever it was, they said, it would likely kill her within a year or two.
Tía Dora's sisters in New Jersey—Hernández's mother and two other aunts—weren't about to let that happen. They pooled their savings and flew her to New York City, where a doctor at Columbia-Presbyterian Medical Center with a penchant for obscure ailments provided a diagnosis: Chagas disease. Transmitted by the bite of triatomine insects, commonly known as kissing bugs, Chagas is endemic in many parts of Latin America. It's caused by the parasite Trypanoma cruzi, which usually settles in the heart, where it feeds on muscle tissue. In some cases, however, it attacks the intestines or esophagus. Tía Dora belonged to that minority.
In 1980, U.S. immigration laws were more forgiving than they are today. Tía Dora was able to have surgery to remove a part of her colon, despite not being a citizen or having a green card. She eventually married a legal resident and began teaching Spanish at an elementary school. Over the next three decades, she earned a graduate degree, built a career, and was widowed. Meanwhile, Chagas continued its slow devastation. "Every couple of years, we were back in the hospital with her," Hernández recalls. "When I was in high school, she started feeling like she couldn't swallow anything. It was the parasite, destroying the muscles of her esophagus."
When Tía Dora died in 2010, at 59, her niece was among the family members at her bedside. By then, Hernández had become a journalist and fiction writer. Researching a short story about Chagas disease, she discovered that it affected an estimated 6 million people in South America, Central America, and Mexico—as well as 300,000 in the United States, most of whom were immigrants from those places. "I was shocked to learn it wasn't rare," she says. "That made me hungry to know more about this disease, and about the families grappling with it."
Hernández's curiosity led her to write The Kissing Bug, a lyrical hybrid of memoir and science reporting that was published in June. It also led her to another revelation: Chagas is not unique. It's among the many maladies that global health experts refer to as neglected tropical diseases—often-disabling illnesses that afflict 1.7 billion people worldwide, while getting notably less attention than the "big three" of HIV/AIDs, malaria, and tuberculosis. NTDs cause fewer deaths than those plagues, but they wreak untold suffering and economic loss.
Shortly before Hernández's book hit the shelves, the World Health Organization released its 2021-2030 roadmap for fighting NTDs. The plan sets targets for controlling, eliminating, or eradicating all the diseases on the WHO's list, through measures ranging from developing vaccines to improving healthcare infrastructure, sanitation, and access to clean water. Experts agree that for the campaign to succeed, leadership from wealthy nations—particularly the United States—is essential. But given the inward turn of many such countries in recent years (evidenced in movements ranging from America First to Brexit), and the continuing urgency of the COVID-19 crisis, public support is far from guaranteed.
As Hernández writes: "It is easier to forget a disease that cannot be seen." NTDs primarily affect residents of distant lands. They kill only 80,000 people a year, down from 204,000 in 1990. So why should Americans to bother to look?
Breaking the circle of poverty and disease
The World Health Organization counts 20 diseases as NTDs. Along with Chagas, they include dengue and chikungunya, which cause high fevers and agonizing pain; elephantiasis, which deforms victims' limbs and genitals; onchocerciasis, which causes blindness; schistosomiasis, which can damage the heart, lungs, brain, and genitourinary system; helminths such as roundworm and whipworm, which cause anemia, stunted growth, and cognitive disabilities; and a dozen more. Such ailments often co-occur in the same patient, exacerbating each other's effects and those of illnesses such as malaria.
NTDs may be spread by insects, animals, soil, or tainted water; they may be parasitic, bacterial, viral, or—in the case of snakebite envenoming—non-infectious. What they have in common is their longtime neglect by public health agencies and philanthropies. In part, this reflects their typically low mortality rates. But the biggest factor is undoubtedly their disempowered patient populations.
"These diseases occur in the setting of poverty, and they cause poverty, because of their chronic and debilitating effects," observes Peter Hotez, dean of the National School of Tropical Medicine at Baylor University and co-director of the Texas Children's Hospital for Vaccine Development. And historically, the everyday miseries of impoverished people have seldom been a priority for those who set the global health agenda.
That began to change about 20 years ago, when Hotez and others developed the conceptual framework for NTDs and early proposals for combating them. The WHO released its first roadmap in 2012, targeting 17 NTDs for control, elimination, or eradication by 2020. (Rabies, snakebite, and dengue were added later.) Since then, the number of people at risk for NTDs has fallen by 600 million, and 42 countries have eliminated at least one such disease. Cases of dracunculiasis—known as Guinea worm disease, for the parasite that creates painful blisters in a patient's skin—have dropped from the millions to just 27 in 2020.
Yet the battle is not over, and the COVID-19 pandemic has disrupted prevention and treatment programs around the globe.
A new direction — and longstanding obstacles
The WHO's new roadmap sets even more ambitious goals for 2030. Among them: reducing by 90 percent the number of people requiring treatment for NTDs; eliminating at least one NTD in another 100 countries; and fully eradicating dracunculiasis and yaws, a disfiguring skin infection.
The plan also places an increased focus on "country ownership," relying on nations with high incidence of NTDs to design their own plans based on local expertise. "I was so excited to see that," says Kristina Talbert-Slagle, director of the Yale College Global Health Studies program. "No one is a better expert on how to address these situations than the people who deal with it day by day."
Another fresh approach is what the roadmap calls "cross-cutting" targets. "One of the really cool things about the plan is how much it emphasizes coordination among different sectors of the health system," says Claire Standley, a faculty member at Georgetown University's Center for Global Health Science and Security. "For example, it explicitly takes into account the zoonotic nature of many neglected tropical diseases—the fact that we have to think about animal health as well as human health when we tackle NTDs."
Whether this grand vision can be realized, however, will depend largely on funding—and that, in turn, is a question of political will in the countries most able to provide it. On the upside, the U.S. has ended its Trump-era feud with the WHO. "One thing that's been really encouraging," says Standley, "has been the strong commitment toward global cooperation from the current administration." Even under the previous president, the U.S. remained the single largest contributor to the global health kitty, spending over $100 million annually on NTDs—six times the figure in 2006, when such financing started.
On the downside, America's outlay has remained flat for several years, and the Biden administration has so far not moved to increase it. A "back-of-the-envelope calculation," says Hotez, suggests that the current level of aid could buy medications for the most common NTDs for about 200 million people a year. But the number of people who need treatment, he notes, is at least 750 million.
Up to now, the United Kingdom—long the world's second-most generous health aid donor—has taken up a large portion of the slack. But the UK last month announced deep cuts in its portfolio, eliminating 102 previously supported countries and leaving only 34. "That really concerns me," Hotez says.
The struggle for funds, he notes, is always harder for projects involving NTDs than for those aimed at higher-profile diseases. His lab, which he co-directs with microbiologist Maria Elena Bottazzi, started developing a COVID-19 vaccine soon after the pandemic struck, for example, and is now in Phase 3 trials. The team has been working on vaccines for Chagas, hookworm, and schistosomiasis for much longer, but trials for those potential game-changers lag behind. "We struggle to get the level of resources needed to move quickly," Hotez explains.
Two million reasons to care
One way to prompt a government to open its pocketbook is for voters to clamor for action. A longtime challenge with NTDs, however, has been getting people outside the hardest-hit countries to pay attention.
The reasons to care, global health experts argue, go beyond compassion. "When we have high NTD burden," says Talbert-Slagle, "it can prevent economic growth, prevent innovation, lead to more political instability." That, in turn, can lead to wars and mass migration, affecting economic and political events far beyond an affected country's borders.
Like Hernández's aunt Dora, many people driven out of NTD-wracked regions wind up living elsewhere. And that points to another reason to care about these diseases: Some of your neighbors might have them. In the U.S., up to 14 million people suffer from neglected parasitic infections—including 70,000 with Chagas in California alone.
When Hernández was researching The Kissing Bug, she worried that such statistics would provide ammunition to racists and xenophobes who claim that immigrants "bring disease" or exploit overburdened healthcare systems. (This may help explain some of the stigma around NTDs, which led Tía Dora to hide her condition from most people outside her family.) But as the book makes clear, these infections know no borders; they flourish wherever large numbers of people lack access to resources that most residents of rich countries take for granted.
Indeed, far from gaming U.S. healthcare systems, millions of low-income immigrants can't access them—or must wait until they're sick enough to go to an emergency room. Since Congress changed the rules in 1996, green card holders have to wait five years before they can enroll in Medicaid. Undocumented immigrants can never qualify.
Closing the great divide
Hernández uses a phrase borrowed from global health crusader Paul Farmer to describe this access gap: "the great epi divide." On one side, she explains, "people will die from cancer, from diabetes, from chronic illnesses later in life. On the other side of the epidemiological divide, people are dying because they can't get to the doctor, or they can't get medication. They don't have a hospital anywhere near them. When I read Dr. Farmer's work, I realized how much that applied to neglected diseases as well."
When it comes to Chagas disease, she says, the epi divide is embodied in the lack of a federal mandate for prenatal or newborn screening. Each year, according to the Centers for Disease Control and Prevention, up to 300 babies in the U.S. are born with Chagas, which can be passed from the mother in utero. The disease can be cured with medication if treated in infancy. (It can also be cured in adults in the acute stage, but is seldom detected in time.) Yet the CDC does not require screening for Chagas—even though newborns are tested for 15 diseases that are less common. According to one study, it would be 10 times cheaper to screen and treat babies and their mothers than to cover the costs related to the illness in later years. Few states make the effort.
The gap that enables NTDs to persist, Hernández argues, is the same one that has led to COVID-19 death rates in Black and Latinx communities that are double those elsewhere in America. To close it, she suggests, caring is not enough.
"When I was working on my book," she says, "I thought about HIV in the '80s, when it had so much stigma that no one wanted to talk about it. Then activists stepped up and changed the conversation. I thought a lot about breast cancer, which was stigmatized for years, until people stepped forward and started speaking out. I thought about Lyme disease. And it wasn't only patients—it was also allies, right? The same thing needs to happen with neglected diseases around the world. Allies need to step up and make demands on policymakers. We need to make some noise."
Nobel Prize goes to technology for mRNA vaccines
Katalin Karikó, pictured, and Drew Weissman won the Nobel Prize for advances in mRNA research that led to the first Covid vaccines.
When Drew Weissman received a call from Katalin Karikó in the early morning hours this past Monday, he assumed his longtime research partner was calling to share a nascent, nagging idea. Weissman, a professor of medicine at the Perelman School of Medicine at the University of Pennsylvania, and Karikó, a professor at Szeged University and an adjunct professor at UPenn, both struggle with sleep disturbances. Thus, middle-of-the-night discourses between the two, often over email, has been a staple of their friendship. But this time, Karikó had something more pressing and exciting to share: They had won the 2023 Nobel Prize in Physiology or Medicine.
The work for which they garnered the illustrious award and its accompanying $1,000,000 cash windfall was completed about two decades ago, wrought through long hours in the lab over many arduous years. But humanity collectively benefited from its life-saving outcome three years ago, when both Moderna and Pfizer/BioNTech’s mRNA vaccines against COVID were found to be safe and highly effective at preventing severe disease. Billions of doses have since been given out to protect humans from the upstart viral scourge.
“I thought of going somewhere else, or doing something else,” said Katalin Karikó. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
Unlocking the power of mRNA
Weissman and Karikó unlocked mRNA vaccines for the world back in the early 2000s when they made a key breakthrough. Messenger RNA molecules are essentially instructions for cells’ ribosomes to make specific proteins, so in the 1980s and 1990s, researchers started wondering if sneaking mRNA into the body could trigger cells to manufacture antibodies, enzymes, or growth agents for protecting against infection, treating disease, or repairing tissues. But there was a big problem: injecting this synthetic mRNA triggered a dangerous, inflammatory immune response resulting in the mRNA’s destruction.
While most other researchers chose not to tackle this perplexing problem to instead pursue more lucrative and publishable exploits, Karikó stuck with it. The choice sent her academic career into depressing doldrums. Nobody would fund her work, publications dried up, and after six years as an assistant professor at the University of Pennsylvania, Karikó got demoted. She was going backward.
“I thought of going somewhere else, or doing something else,” Karikó told Stat in 2020. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
A tale of tenacity
Collaborating with Drew Weissman, a new professor at the University of Pennsylvania, in the late 1990s helped provide Karikó with the tenacity to continue. Weissman nurtured a goal of developing a vaccine against HIV-1, and saw mRNA as a potential way to do it.
“For the 20 years that we’ve worked together before anybody knew what RNA is, or cared, it was the two of us literally side by side at a bench working together,” Weissman said in an interview with Adam Smith of the Nobel Foundation.
In 2005, the duo made their 2023 Nobel Prize-winning breakthrough, detailing it in a relatively small journal, Immunity. (Their paper was rejected by larger journals, including Science and Nature.) They figured out that chemically modifying the nucleoside bases that make up mRNA allowed the molecule to slip past the body’s immune defenses. Karikó and Weissman followed up that finding by creating mRNA that’s more efficiently translated within cells, greatly boosting protein production. In 2020, scientists at Moderna and BioNTech (where Karikó worked from 2013 to 2022) rushed to craft vaccines against COVID, putting their methods to life-saving use.
The future of vaccines
Buoyed by the resounding success of mRNA vaccines, scientists are now hurriedly researching ways to use mRNA medicine against other infectious diseases, cancer, and genetic disorders. The now ubiquitous efforts stand in stark contrast to Karikó and Weissman’s previously unheralded struggles years ago as they doggedly worked to realize a shared dream that so many others shied away from. Katalin Karikó and Drew Weissman were brave enough to walk a scientific path that very well could have ended in a dead end, and for that, they absolutely deserve their 2023 Nobel Prize.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Scientists turn pee into power in Uganda
With conventional fuel cells as their model, researchers learned to use similar chemical reactions to make a fuel from microbes in pee.
At the edge of a dirt road flanked by trees and green mountains outside the town of Kisoro, Uganda, sits the concrete building that houses Sesame Girls School, where girls aged 11 to 19 can live, learn and, at least for a while, safely use a toilet. In many developing regions, toileting at night is especially dangerous for children. Without electrical power for lighting, kids may fall into the deep pits of the latrines through broken or unsteady floorboards. Girls are sometimes assaulted by men who hide in the dark.
For the Sesame School girls, though, bright LED lights, connected to tiny gadgets, chased the fears away. They got to use new, clean toilets lit by the power of their own pee. Some girls even used the light provided by the latrines to study.
Urine, whether animal or human, is more than waste. It’s a cheap and abundant resource. Each day across the globe, 8.1 billion humans make 4 billion gallons of pee. Cows, pigs, deer, elephants and other animals add more. By spending money to get rid of it, we waste a renewable resource that can serve more than one purpose. Microorganisms that feed on nutrients in urine can be used in a microbial fuel cell that generates electricity – or "pee power," as the Sesame girls called it.
Plus, urine contains water, phosphorus, potassium and nitrogen, the key ingredients plants need to grow and survive. Human urine could replace about 25 percent of current nitrogen and phosphorous fertilizers worldwide and could save water for gardens and crops. The average U.S. resident flushes a toilet bowl containing only pee and paper about six to seven times a day, which adds up to about 3,500 gallons of water down per year. Plus cows in the U.S. produce 231 gallons of the stuff each year.
Pee power
A conventional fuel cell uses chemical reactions to produce energy, as electrons move from one electrode to another to power a lightbulb or phone. Ioannis Ieropoulos, a professor and chair of Environmental Engineering at the University of Southampton in England, realized the same type of reaction could be used to make a fuel from microbes in pee.
Bacterial species like Shewanella oneidensis and Pseudomonas aeruginosa can consume carbon and other nutrients in urine and pop out electrons as a result of their digestion. In a microbial fuel cell, one electrode is covered in microbes, immersed in urine and kept away from oxygen. Another electrode is in contact with oxygen. When the microbes feed on nutrients, they produce the electrons that flow through the circuit from one electrod to another to combine with oxygen on the other side. As long as the microbes have fresh pee to chomp on, electrons keep flowing. And after the microbes are done with the pee, it can be used as fertilizer.
These microbes are easily found in wastewater treatment plants, ponds, lakes, rivers or soil. Keeping them alive is the easy part, says Ieropoulos. Once the cells start producing stable power, his group sequences the microbes and keeps using them.
Like many promising technologies, scaling these devices for mass consumption won’t be easy, says Kevin Orner, a civil engineering professor at West Virginia University. But it’s moving in the right direction. Ieropoulos’s device has shrunk from the size of about three packs of cards to a large glue stick. It looks and works much like a AAA battery and produce about the same power. By itself, the device can barely power a light bulb, but when stacked together, they can do much more—just like photovoltaic cells in solar panels. His lab has produced 1760 fuel cells stacked together, and with manufacturing support, there’s no theoretical ceiling, he says.
Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofit into urban wastewater utilities.
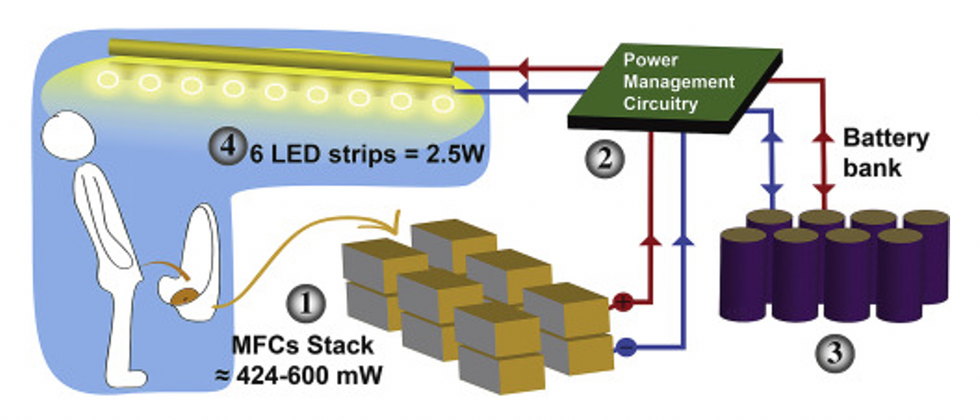
This image shows how the pee-powered system works. Pee feeds bacteria in the stack of fuel cells (1), which give off electrons (2) stored in parallel cylindrical cells (3). These cells are connected to a voltage regulator (4), which smooths out the electrical signal to ensure consistent power to the LED strips lighting the toilet.
Courtesy Ioannis Ieropoulos
Key to the long-term success of any urine reclamation effort, says Orner, is avoiding what he calls “parachute engineering”—when well-meaning scientists solve a problem with novel tech and then abandon it. “The way around that is to have either the need come from the community or to have an organization in a community that is committed to seeing a project operate and maintained,” he says.
Success with urine reclamation also depends on the economy. “If energy prices are low, it may not make sense to recover energy,” says Orner. “But right now, fertilizer prices worldwide are generally pretty high, so it may make sense to recover fertilizer and nutrients.” There are obstacles, too, such as few incentives for builders to incorporate urine recycling into new construction. And any hiccups like leaks or waste seepage will cost builders money and reputation. Right now, Orner says, the risks are just too high.
Despite the challenges, Ieropoulos envisions a future in which urine is passed through microbial fuel cells at wastewater treatment plants, retrofitted septic tanks, and building basements, and is then delivered to businesses to use as agricultural fertilizers. Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofitted into urban wastewater utilities where they can make electricity from the effluent. And unlike solar cells, which are a common target of theft in some areas, nobody wants to steal a bunch of pee.
When Ieropoulos’s team returned to wrap up their pilot project 18 months later, the school’s director begged them to leave the fuel cells in place—because they made a major difference in students’ lives. “We replaced it with a substantial photovoltaic panel,” says Ieropoulos, They couldn’t leave the units forever, he explained, because of intellectual property reasons—their funders worried about theft of both the technology and the idea. But the photovoltaic replacement could be stolen, too, leaving the girls in the dark.
The story repeated itself at another school, in Nairobi, Kenya, as well as in an informal settlement in Durban, South Africa. Each time, Ieropoulos vowed to return. Though the pandemic has delayed his promise, he is resolute about continuing his work—it is a moral and legal obligation. “We've made a commitment to ourselves and to the pupils,” he says. “That's why we need to go back.”
Urine as fertilizer
Modern day industrial systems perpetuate the broken cycle of nutrients. When plants grow, they use up nutrients the soil. We eat the plans and excrete some of the nutrients we pass them into rivers and oceans. As a result, farmers must keep fertilizing the fields while our waste keeps fertilizing the waterways, where the algae, overfertilized with nitrogen, phosphorous and other nutrients grows out of control, sucking up oxygen that other marine species need to live. Few global communities remain untouched by the related challenges this broken chain create: insufficient clean water, food, and energy, and too much human and animal waste.
The Rich Earth Institute in Vermont runs a community-wide urine nutrient recovery program, which collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms.
One solution to this broken cycle is reclaiming urine and returning it back to the land. The Rich Earth Institute in Vermont is one of several organizations around the world working to divert and save urine for agricultural use. “The urine produced by an adult in one day contains enough fertilizer to grow all the wheat in one loaf of bread,” states their website.
Notably, while urine is not entirely sterile, it tends to harbor fewer pathogens than feces. That’s largely because urine has less organic matter and therefore less food for pathogens to feed on, but also because the urinary tract and the bladder have built-in antimicrobial defenses that kill many germs. In fact, the Rich Earth Institute says it’s safe to put your own urine onto crops grown for home consumption. Nonetheless, you’ll want to dilute it first because pee usually has too much nitrogen and can cause “fertilizer burn” if applied straight without dilution. Other projects to turn urine into fertilizer are in progress in Niger, South Africa, Kenya, Ethiopia, Sweden, Switzerland, The Netherlands, Australia, and France.
Eleven years ago, the Institute started a program that collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms. By 2021, the program included 180 donors producing over 12,000 gallons of urine each year. This urine is helping to fertilize hay fields at four partnering farms. Orner, the West Virginia professor, sees it as a success story. “They've shown how you can do this right--implementing it at a community level scale."

