New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
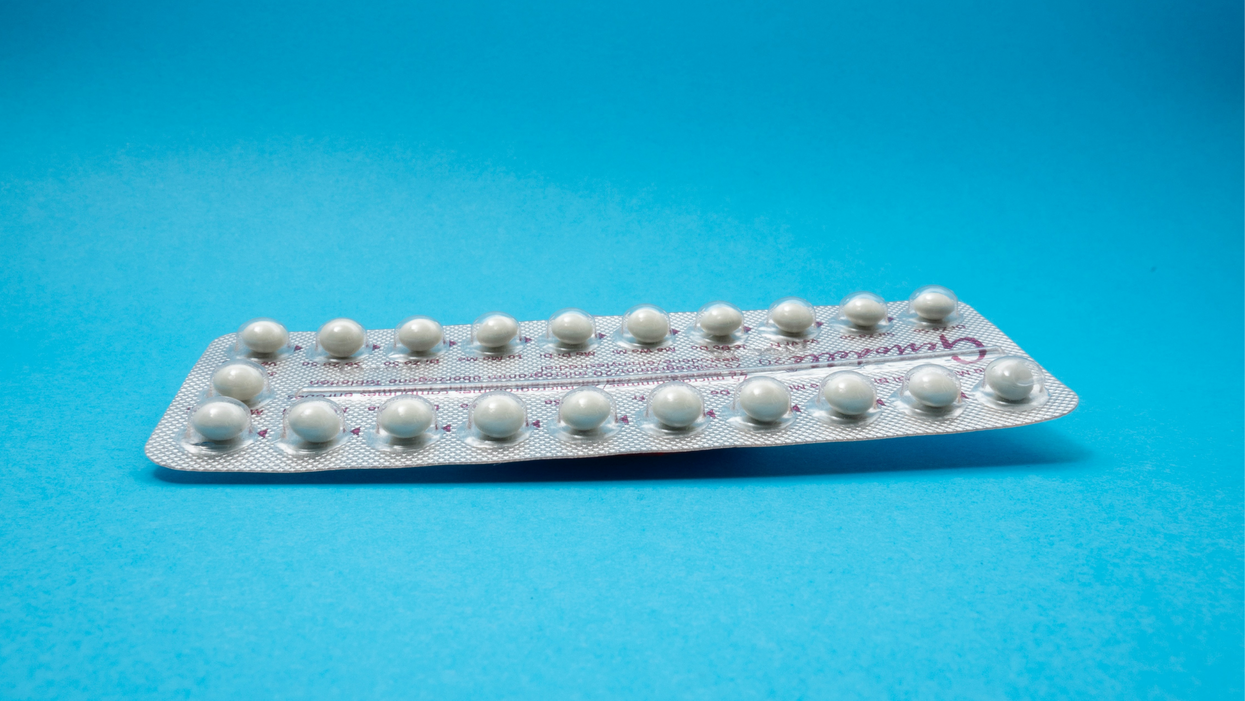
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
“You First”: Who Will Be Front in Line to Get a COVID Vaccine?
A small subset of hospitals and institutions in high-risk areas are likely to go first, according to bioethicist Arthur Caplan.
There is a huge amount riding on the discovery of a vaccine effective against the Covid-19 virus.
Making 660 million of anything without a glitch is—to put it mildly—a tall order in a nation that remains short on masks, gowns, and diagnostic tests despite months of trying to meet demand.
The world is waiting for a vaccine that can liberate everyone from the constraints on liberty required by existing efforts to fight the virus with public health measures such as masks, isolation, and quarantining. President Trump, for the most part, has rejected tough public health measures. Instead he has staked his political future and those of the governors and Congressional Republicans who have followed his lead on delivering a vaccine before Election Day as the solution to the COVID-19 pandemic in the USA. Many scientific experts have been sounding encouraging notes about having a vaccine by the end of this year or early next, as have many CEOs among the more than 160 companies chasing various strategies to identify a safe and effective vaccine.
But the reality is that no matter how fast a vaccine appears, those who might benefit will face a significant period of time before they could receive one. This is due to a variety of realities. Any vaccine faces various regulatory hurdles to insure safety and efficacy. This means completing large-scale studies in tens of thousands of subjects hoping for enough cases of blunted natural infection versus a large placebo control group to determine that a vaccine works. And that takes time--plus adding in delays in manufacturing and delivery, which will create logjams for most prospective recipients.
Shipping is not going to be easy with cold chain storage requirements from -20 to -70 degrees Celsius, from factory to a doctor's office, depending on the vaccine. In addition, many of the vaccines under development require two doses--that is 660 million shots to cover just those in the United States. Making 660 million of anything without a glitch is—to put it mildly—a tall order in a nation that remains short on masks, gowns, and diagnostic tests, despite months of trying to meet demand.
There are three scenarios under which a vaccine can appear but without being in any way available to all Americans.
The first is a vaccine under development in the USA or with some USA financing begins to show promise before a full clinical trial is completed. Current vaccine trials are supervised by Data Safety and Monitoring Boards and those committees could tell a CEO eager to be first to market that their vaccine is looking good at the study's half-way point.
The CEO and vaccine manufacturing company's board then let the White House know that a magic bullet which can ensure the President's reelection is in hand. The President, as he has done many times with other COVID treatments, most recently convalescent plasma, intervenes with the FDA and demands approval using an Emergency Use Authorization, or invoking the Federal Right to Try law he and Mike Pence are constantly touting. FDA Commissioner Steve Hahn folds and an extremely limited supply of vaccine, maybe only 100,000 doses, is available just before Election Day.
The second scenario is that another nation discovers a vaccine that looks safe and effective and the USA is able to buy some supply of it. But again, we are likely, initially, to get an extremely limited amount.
Lastly, the vaccine is approved in a standard manner. A full randomized trial is done, the endpoints are met, and no serious adverse events are identified. It is a USA-funded vaccine so most of it is coming here first. Still the vials and needles and plugs need to be quality-controlled and shipped and stored at the right temperatures. Information sheets and consent forms need to be readied, offered, and signed. Odds are you won't see any of this vaccine until late next year. So, who is going to get the first shots?
Some people under all of these scenarios are going to say, "Count me out." They don't trust vaccines or they don't trust the government to provide a safe one. Others may say, "The first one out of the box may be OK, but I am going to wait for the 'best' one before I take one." Even if those numbers are large, it is still certain that there will be more takers than can be vaccinated.
If you look at the discussion of vaccine rationing, almost everybody — including government officials, FDA officials, advisory panelists and ethicists — says the first group that should get vaccinated are at-risk healthcare workers. They say it, although they're not always clear about why.
One reason is that you need to give it to health care workers first because they will keep the healthcare system going. Another is that you need to give it to them first because they face more risk and they should get rewarded for having done and continuing to do that -- their bravery ought to be rewarded and their risk reduced.
A subset of hospitals and institutions in high risk areas will [go first] and that will be it for a significant period of time.
Both of these arguments for health care worker priority are not completely convincing. Food and power and vaccine manufacturing are arguably as important as health care, but workers in those areas don't get priority attention in most guidelines. And many Americans face risks from COVID comparable to health care workers, especially those who are not on the front lines in ERs and ICUs. Prisoners, military personnel who work on warships, the elderly, nursing home residents, and poor minorities are disproportionately affected by COVID. However, none of them are going first, nor is it clear how to weigh their claims in competing against one another for a scarce vaccine.
But, there's something else that's interesting in deciding who goes first. When people all agree, as they almost always do, that it's health care workers who must go first, a huge problem remains. What is the definition of who's a healthcare worker? You could easily get millions and millions of people designated as healthcare workers who would have a claim to go first.
We normally think that health care worker means doctors and nurses. But, if we go beyond those who work in ERs and ICUs, the number is big. And we must, because no ER or ICU can run without huge numbers of supporting individuals.
If you don't vaccinate lab technicians, people who clean the rooms, make food, transport patients, provide security, do the laundry, run the IT, students, volunteers and so on, you're not going to have a functioning hospital. If you don't include those working in nursing homes, home care and hospices along with those making and supplying vital equipment and bringing in patients via ambulances, police cars, and fire trucks, you don't have a functioning ICU, much less a health care system.
The total number involved could easily exceed tens of millions depending on how broadly the definition is set.
So, what is likely to happen is that health care workers will not go first. A subset of hospitals and institutions in high risk areas will and that will be it for a significant period of time. Health care institutions in hot spots, plus the supporting services they need will go first and then vaccine availability will slowly expand to other health care institutions and the essential workers needed to keep them functioning. Then consideration will also be given to how best to control the spread of the virus in selecting hot spots versus saving prisoners or the poor. And you can be sure, whatever the guidelines are, that the military and security folks will demand their share.
For many, many months if not a year or more, most people will not have to face a choice about vaccinating. The supply just won't be there for the general public. It is a small sample of high-risk health care workers including vaccine manufacturing employees and shippers, plus essential workers to keep hospitals and nursing homes going, who will be first in line. Odds are you and your family will still be wearing masks and social distancing well into next year.
Virtual clinical trials are helping to eradicate logistical barriers to clinical trial participation, though trust is still an issue.
Herman Taylor, director of the cardiovascular research institute at Morehouse college, got in touch with UnitedHealth Group early in the pandemic.
The very people who most require solutions to COVID are those who are least likely to be involved in the search to find them.
A colleague he worked with at Grady Hospital in Atlanta was the guy when it came to studying sickle cell disease, a recessive genetic disorder that causes red blood cells to harden into half-moon shapes, causing cardiovascular problems. Sickle cell disease is more common in African Americans than it is in Caucasians, in part because having just one gene for the disease, called sickle cell trait, is protective against malaria, which is endemic to much of Africa. Roughly one in 12 African Americans carry sickle cell trait, and Taylor's colleague wondered if this could be one factor affecting differential outcomes for COVID-19.
UnitedHealth Group granted Taylor and his colleague the money to study sickle cell trait in COVID, and then, as they continued working together, they began to ask Taylor his opinion on other topics. As an insurance company, United had realized early in the pandemic that it was sitting on a goldmine of patient data—some 120 million patients' worth—that it could sift through to look for potential COVID treatments.
Their researchers thought they had found one: In a small subset of 14,000 people who'd contracted COVID, there was a group whose bills were paid by Medicare (which the researchers took as a proxy for older age). The people in this group who were taking ACE inhibitors, blood vessel dilators often used to treat high blood pressure, were 40 percent less likely to be hospitalized than those who were not taking the drug.
The connection between ACE inhibitors and COVID hospitalizations was a correlation, a statistical association. To determine whether the drugs had any real effect on COVID outcomes, United would have to perform another, more rigorous study. They would have to assign some people to receive small doses of ACE inhibitors, and others to receive placebos, and measure the outcomes under each condition. They planned to do this virtually, allowing study participants to sign up and be screened online, and sending drugs, thermometers, and tests through the mail. There were two reasons to do it this way: First, the U.S. Food and Drug Administration had been advising medical researchers to embrace new strategies in clinical trials as a way to protect participants during the pandemic.
The second reason was why they asked Herman Taylor to co-supervise it: Clinical trials have long had a diversity problem. And going virtual is a potential solution.
Since the beginning of the pandemic, COVID-19 has infected people of color at a rate of three times that of Caucasians (killing Black people at a rate 2.5 times as high, and Hispanic and American Indian or Alaska Native people at a rate 1.3 times as high). A number of explanations have been put forth to explain this disproportionate toll. Among them: higher levels of poverty, essential jobs that increase exposure, and lower quality or inadequate access to medical care.
Unfortunately, these same factors also affect who participates in research. People of color may be less likely to have doctors recommend studies to them. They may not have the time or the resources to hang out in a waiting room for hours. They may not live near large research institutions that conduct trials. The result is that new treatments, even for diseases that affect Latin, Native American, or African American populations in greater proportions, are studied mostly in white volunteers. The very people who most require solutions to COVID are those who are least likely to be involved in the search to find them.
Virtual trials can alleviate a number of these problems. Not only can people find and request to participate in these types of trials through their phones or computers, virtual trials also cover more costs, include a larger geographical range, and have inherently flexible hours.
"[In a traditional study] you have to go to a doctor's office to enroll and drive a couple of hours and pay $20 for parking and pay $15 for a sandwich in the hospital cafeteria and arrange for daycare for your kids and take time off of work," says Dr. Jonathan Cotliar, chief medical officer of Science37, a platform that investigators can hire to host and organize their trials virtually. "That's a lot just for one visit, much less over the course of a six to 12-month study."
Cotliar's data suggests that virtual trials' enhanced access seriously affects the racial makeup of a given study's participant pool. Sixty percent of patients enrolled in Science37 trials are non-Caucasian, which is, Cotliar says, "staggering compared to what you find in traditional site-based research."
But access is not the only barrier to including more people of color in clinical trials. There is also trust. When agreeing to sign up for research, undocumented immigrants may worry about finding themselves in legal trouble or without any medical support should something go wrong. In a country with a history of experimenting on African Americans without their consent, black people may not trust institutions not to use them as guinea pigs.
"A lot of people report being somewhat disregarded or disrespected once entering the healthcare system," Taylor says. "You take it all together, then people wonder, well, okay, this is how the system tends to regard me, yet now here come these people doing research, and they're all about getting me into their studies." Not so surprising that a lot of people may respond with a resounding "No thanks."
United's ACE inhibitor trial was notable for addressing both of these challenges. In addition to covering costs and allowing study subjects to participate from their own homes, it was being co-sponsored by a professor at Morehouse, one of the country's historic black colleges and universities (often abbreviated HBCUs). United was recruiting heavily in Atlanta, whose population is 52 percent African American. The study promised a thoughtful introduction to a more egalitarian future of medical research.
There's just one problem: It isn't going to happen.
This month, in preparation for the study, United reanalyzed their ACE inhibitor data with all the new patients who'd contracted COVID in the months since their first analysis. Their original data set had been concentrated in the Northeast, mostly New York City, where the earliest outbreak took place. In the 12 weeks it had taken them to set up the virtual followup study, epicenters had shifted. United's second, more geographically comprehensive sample had ten times the number of people in it. And in that sample, the signal simply disappeared.
"I was shocked, but that's the reality," says Deneen Vojta, executive vice president of enterprise research and development for UnitedHealth Group. "You make decisions based on the data, but when you get more data, more information, you might make a different decision. The answer is the answer."
There was no point in running a virtual ACE inhibitor study if a larger, more representative sample of people indicated the drug was unlikely to help anyone. Still, the model United had established to run the trial remains viable. Even as she scrapped the ACE inhibitor study, Vojta hoped not just to continue United's relationship with Dr. Taylor and Morehouse, but to formalize it. Virtual platforms are still an important part of their forthcoming trials.
If people don't believe a vaccine has been created with them in mind, then they won't take it, and it won't matter whether it exists or not.
United is not alone in this approach. As phase three trials for vaccines against SARS CoV-2 get underway, big pharma companies have been publicly articulating their own strategies for including more people of color in clinical trials, and many of these include virtual elements. Janelle Sabo, global head of clinical innovation, systems and clinical supply chain at Eli Lilly, told me that the company is employing home health and telemedicine, direct-to-patient shipping and delivery, and recruitment using social media and geolocation to expand access to more diverse populations.
Dr. Macaya Douoguih, Head of Clinical Development and Medical Affairs for Janssen Vaccines under Johnson & Johnson, spoke to Congress about this issue during a July hearing before the House Energy and Commerce Oversight and Investigations Subcommittee. She said that the company planned to institute a "focused digital and community outreach plan to provide resources and opportunities to encourage participation in our clinical trials," and had partnered with Johns Hopkins Bloomberg School of Public Health "to understand how the COVID-19 crisis is affecting different communities in the United States."
But while some of these plans are well thought-out, others are concerningly nebulous, featuring big pronouncements but fewer tangible strategies. In that same July hearing, Massachusetts representative Joe Kennedy III (D) sounded like a frustrated teacher when admonishing four of the five leads of the present pharma companies (AstraZeneca, Johnson & Johnson, Merck, Moderna, and Pfizer) for not explaining exactly how they'd ensure diversity both in the study of their vaccines, and in their eventual distribution.
This matters: The uptake of the flu vaccine is 10 percentage points lower in both the African American and Hispanic communities than it is in Caucasians. A Pew research study conducted early in the pandemic found that just 54 percent of Black adults said they "would definitely or probably get a coronavirus vaccine," compared to 74 percent of Whites and Hispanics.
"As a good friend of mine, Dr. [James] Hildreth, president at Meharry, another HBC medical school, likes to say: 'A vaccine is great, but it is the vaccination that saves people,'" Taylor says. If people don't believe a vaccine has been created with them in mind, then they won't take it, and it won't matter whether it exists or not.
In this respect, virtual platforms remain an important first step, at least in expanding admittance. In June, United Health opened up a trial to their entire workforce for a computer game that could treat ADHD. In less than two months, 1,743 people had signed up for it, from all different socioeconomic groups, from all over the country. It was inching closer to the kind of number you need for a phase three vaccine trial, which can require tens of thousands of people. Back when they'd been planning the ACE inhibitor study, United had wanted 9,600 people to agree to participate.
Now, with the help of virtual enrollment, they hope they can pull off similarly high numbers for the COVID vaccine trial they will be running for an as-yet-unnamed pharmaceutical partner. It stands to open in September.

