New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
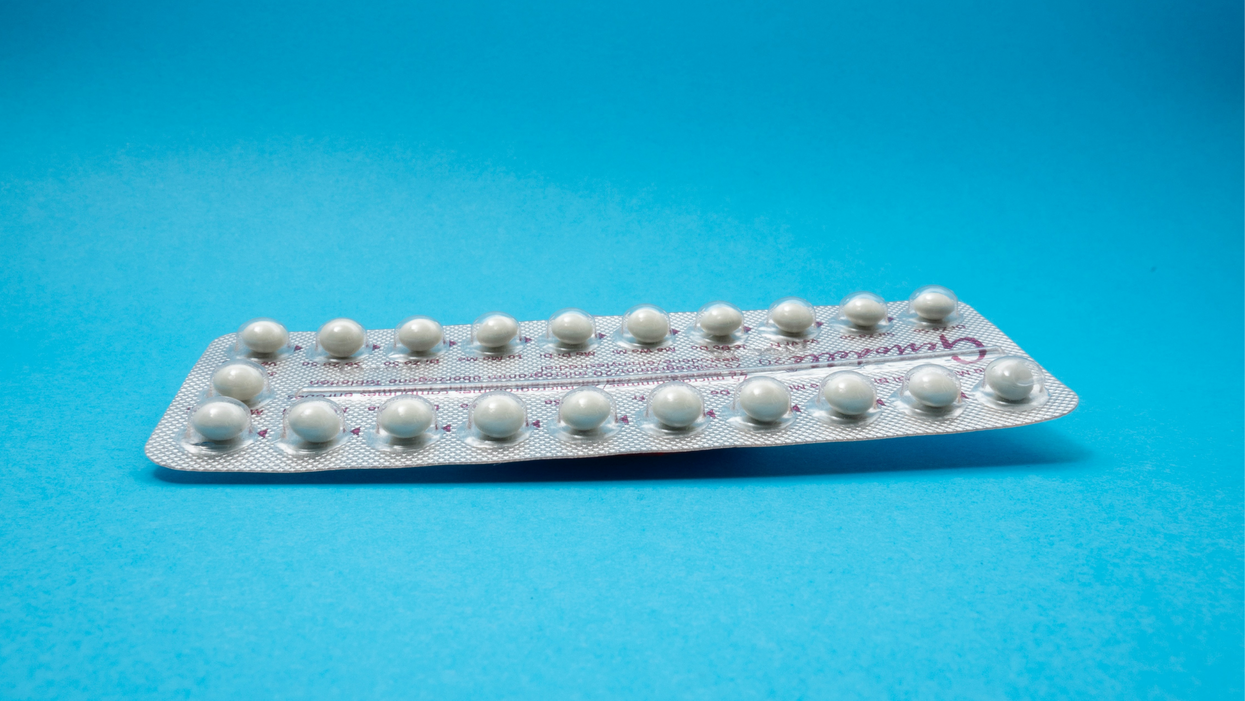
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
A participatory research project has evolved in the world's most powerful networked supercomputer to identify drug targets for COVID-19.
With millions of people left feeling helpless as COVID-19 sweeps across the U.S. and the rest of the planet, there is one way in which absolutely anyone can help fight the pandemic -- all you need is a computer and an Internet connection.
"The more donors that participate, the more science we're able to do."
The Folding@home project allows members of the public to contribute a portion of their computing power to a gigantic virtual network which has mushroomed over the past month to become the most powerful supercomputer on the planet.
As of April 6, more than one million people across the globe have donated some of their home computing resources to the project. Combined, this gives Folding@home processing powers that dwarf even NASA and IBM's most powerful devices. To join, all you have to do is go to this website and click 'Download Now' to load the Folding@home software on your computer. This runs in the background, and only adds your unused computing power to the project, so it will not drain resources from tasks you're trying to do.
"It's totally crazy," said Vincent Voelz, associate professor of chemistry at Temple University, Philadelphia, and one of the scientists leading the project. "A month ago, we had around 30,000 to 40,000 participants. And then last week, it rose up 400,000 and now we've hit a million. But the more donors that participate, the more science we're able to do."
Voelz and the other scientists behind Folding@home are using these vast resources to model the ever-changing shapes of the coronavirus's proteins, in the hopes of identifying vulnerabilities or 'pockets' in its structure that can be targeted with new drugs.
One of the reasons it's difficult to find treatments for viruses like COVID-19 and Ebola is because the proteins, the innate building blocks of the viral structure, have notoriously smooth surfaces, making it hard for drugs to bind to them.
But viral proteins don't stay still. They are constantly evolving and changing shape as the atoms within push and pull against each other. Having a supercomputer enables scientists to simulate all these different shapes, revealing potential weaknesses which were not immediately visible. And the more powerful the supercomputer, the faster these simulations can happen.
"Simulating these protein motions also enables us to answer basic questions such as what makes this new coronavirus strain different from previous strains," said Voelz. "Is there something about the dynamics of these proteins that makes it more virulent?"
Finding a genuinely novel drug for COVID-19 is particularly critical.
Once they have identified suitable pockets within the proteins of COVID-19, the Folding@home scientists can then take the many compounds being identified by chemists around the world as potential drugs, and try to predict which ones will stand the best chance of binding to those pockets and inhibiting the virus's ability to invade and take over human cells.
"We have so much bandwidth now with Folding@home that we really think we can make a dent with screening these, and prioritizing which compounds are then going to get experimentally tested," said Voeltz.
The team are particularly hopeful they can succeed, having already used the supercomputer to identify a new vulnerability in the Ebola virus, which could go on to yield a new treatment for the disease.
Finding a genuinely novel drug for COVID-19 is particularly critical. While researchers are also looking at repurposing existing medications, like the antimalarials Hydroxychloroquine and Chloroquine (which have just been approved by the FDA for emergency use in coronavirus patients), concerns remain about the safety of these treatments. Researchers at the Mayo Clinic recently warned that the use of these drugs could have the side effect of inducing heart problems and run the risk of sudden cardiac arrest.
But with the death toll increasing by the day, speed is of the essence. Voelz explains that the scientific community has been left playing catch-up, because a drug was never actually developed for the original SARS outbreak in the early 2000s. The enormous computational power of the Folding@home project has the potential to allow scientists to quickly answer some of the key questions needed to get a new treatment into the pipeline.
"We don't have a SARS drug for whatever reason," said Voelz. "So the missing ingredient really, is the basic science to reveal possible drug targets and then the pharma can take that information and do the engineering work and optimizing and clinically testing drugs. But we now have a lot of basic science going on in response to this pandemic."
Stefania Sterling with her son Charlie, who was diagnosed at age 3 with autism.
Stefania Sterling was just 21 when she had her son, Charlie. She was young and healthy, with no genetic issues apparent in either her or her husband's family, so she expected Charlie to be typical.
"It is surprising that the prevalence of a significant disorder like autism has risen so consistently over a relatively brief period."
It wasn't until she went to a Mommy and Me music class when he was one, and she saw all the other one-year-olds walking, that she realized how different her son was. He could barely crawl, didn't speak, and made no eye contact. By the time he was three, he was diagnosed as being on the lower functioning end of the autism spectrum.
She isn't sure why it happened – and researchers, too, are still trying to understand the basis of the complex condition. Studies suggest that genes can act together with influences from the environment to affect development in ways that lead to Autism Spectrum Disorder (ASD). But rates of ASD are rising dramatically, making the need to figure out why it's happening all the more urgent.
The Latest News
Indeed, the CDC's latest autism report, released last week, which uses 2016 data, found that the prevalence of ASD in four-year-old children was one in 64 children, or 15.6 affected children per 1,000. That's more than the 14.1 rate they found in 2014, for the 11 states included in the study. New Jersey, as in years past, was the highest, with 25.3 per 1,000, compared to Missouri, which had just 8.8 per 1,000.
The rate for eight-year-olds had risen as well. Researchers found the ASD prevalence nationwide was 18.5 per 1,000, or one in 54, about 10 percent higher than the 16.8 rate found in 2014. New Jersey, again, was the highest, at one in 32 kids, compared to Colorado, which had the lowest rate, at one in 76 kids. For New Jersey, that's a 175 percent rise from the baseline number taken in 2000, when the state had just one in 101 kids.
"It is surprising that the prevalence of a significant disorder like autism has risen so consistently over a relatively brief period," said Walter Zahorodny, an associate professor of pediatrics at Rutgers New Jersey Medical School, who was involved in collecting the data.
The study echoed the findings of a surprising 2011 study in South Korea that found 1 in every 38 students had ASD. That was the the first comprehensive study of autism prevalence using a total population sample: A team of investigators from the U.S., South Korea, and Canada looked at 55,000 children ages 7 to 12 living in a community in South Korea and found that 2.64 percent of them had some level of autism.
Searching for Answers
Scientists can't put their finger on why rates are rising. Some say it's better diagnosis. That is, it's not that more people have autism. It's that we're better at detecting it. Others attribute it to changes in the diagnostic criteria. Specifically, the May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders-5 -- the standard classification of mental disorders -- removed the communication deficit from the autism definition, which made more children fall under that category. Cynical observers believe physicians and therapists are handing out the diagnosis more freely to allow access to services available only to children with autism, but that are also effective for other children.
Alycia Halladay, chief science officer for the Autism Science Foundation in New York, said she wishes there were just one answer, but there's not. While she believes the rising ASD numbers are due in part to factors like better diagnosis and a change in the definition, she does not believe that accounts for the entire rise in prevalence. As for the high numbers in New Jersey, she said the state has always had a higher prevalence of autism compared to other states. It is also one of the few states that does a good job at recording cases of autism in its educational records, meaning that children in New Jersey are more likely to be counted compared to kids in other states.
"Not every state is as good as New Jersey," she said. "That accounts for some of the difference compared to elsewhere, but we don't know if it's all of the difference in prevalence, or most of it, or what."
"What we do know is that vaccinations do not cause autism."
There is simply no defined proven reason for these increases, said Scott Badesch, outgoing president and CEO of the Autism Society of America.
"There are suggestions that it is based on better diagnosis, but there are also suggestions that the incidence of autism is in fact increasing due to reasons that have yet been determined," he said, adding, "What we do know is that vaccinations do not cause autism."
Zahorodny, the pediatrics professor, believes something is going on beyond better detection or evolving definitions.
"Changes in awareness and shifts in how children are identified or diagnosed are relevant, but they only take you so far in accounting for an increase of this magnitude," he said. "We don't know what is driving the surge in autism recorded by the ADDM Network and others."
He suggested that the increase in prevalence could be due to non-genetic environmental triggers or risk factors we do not yet know about, citing possibilities including parental age, prematurity, low birth rate, multiplicity, breech presentation, or C-section delivery. It may not be one, but rather several factors combined, he said.
"Increases in ASD prevalence have affected the whole population, so the triggers or risks must be very widely dispersed across all strata," he added.
There are studies that find new risk factors for ASD almost on a daily basis, said Idan Menashe, assistant professor in the Department of Health at Ben-Gurion University of the Negev, the fastest growing research university in Israel.
"There are plenty of studies that find new genetic variants (and new genes)," he said. In addition, various prenatal and perinatal risk factors are associated with a risk of ASD. He cited a study his university conducted last year on the relationship between C-section births and ASD, which found that exposure to general anesthesia may explain the association.
Whatever the cause, health practitioners are seeing the consequences in real time.
"People say rates are higher because of the changes in the diagnostic criteria," said Dr. Roseann Capanna-Hodge, a psychologist in Ridgefield, CT. "And they say it's easier for children to get identified. I say that's not the truth and that I've been doing this for 30 years, and that even 10 years ago, I did not see the level of autism that I do see today."
Sure, we're better at detecting autism, she added, but the detection improvements have largely occurred at the low- to mid- level part of the spectrum. The higher rates of autism are occurring at the more severe end, in her experience.
A Polarizing Theory
Among the more controversial risk factors scientists are exploring is the role environmental toxins may play in the development of autism. Some scientists, doctors and mental health experts suspect that toxins like heavy metals, pesticides, chemicals, or pollution may interrupt the way genes are expressed or the way endocrine systems function, manifesting in symptoms of autism. But others firmly resist such claims, at least until more evidence comes forth. To date, studies have been mixed and many have been more associative than causative.
"Today, scientists are still trying to figure out whether there are other environmental changes that can explain this rise, but studies of this question didn't provide any conclusive answer," said Menashe, who also serves as the scientific director of the National Autism Research Center at BGU.
"It's not everything that makes Charlie. He's just like any other kid."
That inconclusiveness has not dissuaded some doctors from taking the perspective that toxins do play a role. "Autism rates are rising because there is a mismatch between our genes and our environment," said Julia Getzelman, a pediatrician in San Francisco. "The majority of our evolution didn't include the kinds of toxic hits we are experiencing. The planet has changed drastically in just the last 75 years –- it has become more and more polluted with tens of thousands of unregulated chemicals being used by industry that are having effects on our most vulnerable."
She cites BPA, an industrial chemical that has been used since the 1960s to make certain plastics and resins. A large body of research, she says, has shown its impact on human health and the endocrine system. BPA binds to our own hormone receptors, so it may negatively impact the thyroid and brain. A study in 2015 was the first to identify a link between BPA and some children with autism, but the relationship was associative, not causative. Meanwhile, the Food and Drug Administration maintains that BPA is safe at the current levels occurring in food, based on its ongoing review of the available scientific evidence.
Michael Mooney, President of St. Louis-based Delta Genesis, a non-profit organization that treats children struggling with neurodevelopmental delays like autism, suspects a strong role for epigenetics, which refers to changes in how genes are expressed as a result of environmental influences, lifestyle behaviors, age, or disease states.
He believes some children are genetically predisposed to the disorder, and some unknown influence or combination of influences pushes them over the edge, triggering epigenetic changes that result in symptoms of autism.
For Stefania Sterling, it doesn't really matter how or why she had an autistic child. That's only one part of Charlie.
"It's not everything that makes Charlie," she said. "He's just like any other kid. He comes with happy moments. He comes with sad moments. Just like my other three kids."

