New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
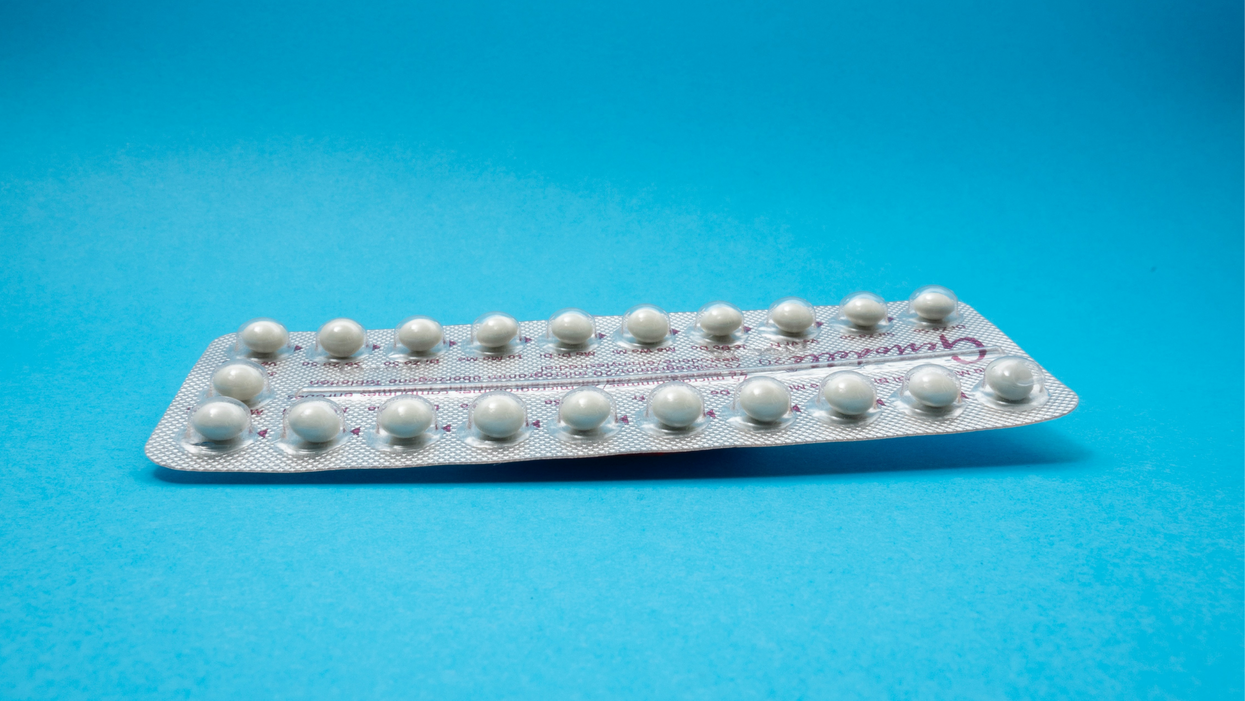
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
Harvard Scientist’s Breakthrough Could Make Humans Resistant to All Viruses
DNA recoding has already made E. coli bacteria immune to 90 percent of viruses that can infect it.
[Ed. Note: We're thrilled to present the first episode in our new Moonshot series, which will explore four cutting-edge scientific developments that stand to fundamentally transform our world.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
In the future, a paper strip reminiscent of a pregnancy test could be used to quickly diagnose the flu and other infectious diseases.
Trying to get a handle on CRISPR news in 2019 can be daunting if you haven't been avidly reading up on it for the last five years.
CRISPR as a diagnostic tool would be a major game changer for medicine and agriculture.
On top of trying to grasp how the science works, and keeping track of its ever expanding applications, you may also have seen coverage of an ongoing legal battle about who owns the intellectual property behind the gene-editing technology CRISPR-Cas9. And then there's the infamous controversy surrounding a scientist who claimed to have used the tool to edit the genomes of two babies in China last year.
But gene editing is not the only application of CRISPR-based biotechnologies. In the future, it may also be used as a tool to diagnose infectious diseases, which could be a major game changer for medicine and agriculture.
How It Works
CRISPR is an acronym for a naturally occurring DNA sequence that normally protects microbes from viruses. It's been compared to a Swiss army knife that can recognize an invader's DNA and precisely destroy it. Repurposed for humans, CRISPR can be paired with a protein called Cas9 that can detect a person's own DNA sequence (usually a problematic one), cut it out, and replace it with a different sequence. Used this way, CRISPR-Cas9 has become a valuable gene-editing tool that is currently being tested to treat numerous genetic diseases, from cancer to blood disorders to blindness.
CRISPR can also be paired with other proteins, like Cas13, which target RNA, the single-stranded twin of DNA that viruses rely on to infect their hosts and cause disease. In a future clinical setting, CRISPR-Cas13 might be used to diagnose whether you have the flu by cutting a target RNA sequence from the virus. That spliced sequence could stick to a paper test strip, causing a band to show up, like on a pregnancy test strip. If the influenza virus and its RNA are not present, no band would show up.
To understand how close to reality this diagnostic scenario is right now, leapsmag chatted with CRISPR pioneer Dr. Feng Zhang, a molecular biologist at the Broad Institute of MIT and Harvard.
What do you think might be the first point of contact that a regular person or patient would have with a CRISPR diagnostic tool?
FZ: I think in the long run it will be great to see this for, say, at-home disease testing, for influenza and other sorts of important public health [concerns]. To be able to get a readout at home, people can potentially quarantine themselves rather than traveling to a hospital and then carrying the risk of spreading that disease to other people as they get to the clinic.
"You could conceivably get a readout during the same office visit, and then the doctor will be able to prescribe the right treatment right away."
Is this just something that people will use at home, or do you also foresee clinical labs at hospitals applying CRISPR-Cas13 to samples that come through?
FZ: I think we'll see applications in both settings, and I think there are advantages to both. One of the nice things about SHERLOCK [a playful acronym for CRISPR-Cas13's longer name, Specific High-sensitivity Enzymatic Reporter unLOCKing] is that it's rapid; you can get a readout fairly quickly. So, right now, what people do in hospitals is they will collect your sample and then they'll send it out to a clinical testing lab, so you wouldn't get a result back until many hours if not several days later. With SHERLOCK, you could conceivably get a readout during the same office visit, and then the doctor will be able to prescribe the right treatment right away.
I just want to clarify that when you say a doctor would take a sample, that's referring to urine, blood, or saliva, correct?
FZ: Right. Yeah, exactly.
Thinking more long term, are there any Holy Grail applications that you hope CRISPR reaches as a diagnostic tool?
FZ: I think in the developed world we'll hopefully see this being used for influenza testing, and many other viral and pathogen-based diseases—both at home and also in the hospital—but I think the even more exciting direction is that this could be used and deployed in parts of the developing world where there isn't a fancy laboratory with elaborate instrumentation. SHERLOCK is relatively inexpensive to develop, and you can turn it into a paper strip test.
Can you quantify what you mean by relatively inexpensive? What range of prices are we talking about here?
FZ: So without accounting for economies of scale, we estimate that it can cost less than a dollar per test. With economy of scale that cost can go even lower.
Is there value in developing what is actually quite an innovative tool in a way that visually doesn't seem innovative because it's reminiscent of a pregnancy test? And I don't mean that as an insult.
FZ: [Laughs] Ultimately, we want the technology to be as accessible as possible, and pregnancy test strips have such a convenient and easy-to-use form. I think modeling after something that people are already familiar with and just changing what's under the hood makes a lot of sense.

Feng Zhang
(Photo credit: Justin Knight, McGovern Institute)
It's probably one of the most accessible at-home diagnostic tools at this point that people are familiar with.
FZ: Yeah, so if people know how to use that, then using something that's very similar to it should make the option very easy.
You've been quite vocal in calling for some pauses in CRISPR-Cas9 research to make sure it doesn't outpace the ethics of establishing pregnancies with that version of the tool. Do you have any concerns about using CRISPR-Cas13 as a diagnostic tool?
I think overall, the reception for CRISPR-based diagnostics has been overwhelmingly positive. People are very excited about the prospect of using this—for human health and also in agriculture [for] detection of plant infections and plant pathogens, so that farmers will be able to react quickly to infection in the field. If we're looking at contamination of foods by certain bacteria, [food safety] would also be a really exciting application.
Do you feel like the controversies surrounding using CRISPR as a gene-editing tool have overshadowed its potential as a diagnostics tool?
FZ: I don't think so. I think the potential for using CRISPR-Cas9 or CRISPR-Cas12 for gene therapy, and treating disease, has captured people's imaginations, but at the same time, every time I talk with someone about the ability to use CRISPR-Cas13 as a diagnostic tool, people are equally excited. Especially when people see the very simple paper strip that we developed for detecting diseases.
Are CRISPR as a gene-editing tool and CRISPR as a diagnostics tool on different timelines, as far as when the general public might encounter them in their real lives?
FZ: I think they are all moving forward quite quickly. CRISPR as a gene-editing tool is already being deployed in human health and agriculture. We've already seen the approval for the development of growing genome-edited mushrooms, soybeans, and other crop species. So I think people will encounter those in their daily lives in that manner.
Then, of course, for disease treatment, that's progressing rapidly as well. For patients who are affected by sickle cell disease, and also by a degenerative eye disease, clinical trials are already starting in those two areas. Diagnostic tests are also developing quickly, and I think in the coming couple of years, we'll begin to see some of these reaching into the public realm.
"There are probably 7,000 genetic diseases identified today, and most of them don't have any way of being treated."
As far its limits, will it be hard to use CRISPR as a diagnostic tool in situations where we don't necessarily understand the biological underpinnings of a disease?
FZ: CRISPR-Cas13, as a diagnostic tool, at least in the current way that it's implemented, is a detection tool—it's not a discovery tool. So if we don't know what we're looking for, then it's going to be hard to develop Cas13 to detect it. But even in the case of a new infectious disease, if DNA sequencing or RNA sequencing information is available for that new virus, then we can very rapidly program a Cas13-based system to detect it, based on that sequence.
What's something you think the public misunderstands about CRISPR, either in general, or specifically as a diagnostic tool, that you wish were better understood?
FZ: That's a good question. CRISPR-Cas9 and CRISPR-Cas12 as gene editing tools, and also CRISPR-Cas13 as a diagnostic tool, are able to do some things, but there are still a lot of capabilities that need to be further developed. So I think the potential for the technology will unfold over the next decade or so, but it will take some time for the full impact of the technology to really get realized in real life.
What do you think that full impact is?
FZ: There are probably 7,000 genetic diseases identified today, and most of them don't have any way of being treated. It will take some time for CRISPR-Cas9 and Cas12 to be really developed for addressing a larger number of those diseases. And then for CRISPR-based diagnostics, I think you'll see the technology being applied in a couple of initial cases, and it will take some time to develop that more broadly for many other applications.