New Options Are Emerging in the Search for Better Birth Control

A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
Electricity is emerging as a powerful treatment for chronic ailments.
Kelly, a case manager for an insurance company, spent years battling both migraines and Crohn's, a disease in which the immune system attacks the intestines.
For many people, like Kelly, a stronger electric boost to the vagus nerve could be life-changing.
After she had her large intestine removed, her body couldn't absorb migraine medication. Last year, about twice a month, she endured migraines so bad she couldn't function. "It would go up to a ten, and I would rock, wait it out," she said. The pain might last for three days.
Then her neurologist showed her a new device, gammaCore, that tames migraines by stimulating a nerve—not medication. "I don't have to put a chemical in my body," she said. "I was thrilled."
At first, Kelly used the device at the onset of a migraine, applying electricity to her pulse at the front of her neck for six minutes. The pain peaked at about half the usual intensity--low enough, she said, that she could go to work. Four months ago, she began using the device for two minutes each night as prevention, and she hasn't had a serious migraine since.
The Department of Defense and Veterans Administration now offer gammaCore to patients, but it hasn't yet been approved by Medicare, Medicaid, or most insurers. A month of therapy costs $600 before insurance or a generous financial assistance program kicks in.

A patient uses gammaCore, a non invasive vagal nerve stimulator device that was FDA approved in November 2018, to treat her migraine.
(Photo captured from a patient video at gammacore.com)
If the poet Walt Whitman wrote "I Sing The Body Electric" today, he might get specific and point to the vagus nerve, a bundle of fibers that run from the brainstem down the neck to the heart and gut. Singing stimulates it—and for many people, like Kelly, a stronger electric boost to the nerve could be life-changing.
The mind-body connection isn't just an idea — the vagus nerve literally carries signals from the mind to the body and back. It may explain the link between childhood trauma and illnesses such as chronic pain and headaches in adults. "How is it possible that a psychological event causes pain years later?" asked Peter Staats, co-founder of electroCore, which has won approval for its new device from the Food and Drug Administration (FDA) for both migraine and cluster headaches. "There has to be a mind-body interface, and that is the vagus nerve," he said.
Scientists knew that this nerve controlled your heart rate and blood pressure, but in the past decade it has been linked to both pain and the immune system.
"Everything is gated through the vagus -- problems with the gut, the heart, and the lungs," said Chris Wilson, a researcher at Loma Linda University, in California. Wilson is studying how vagus nerve stimulation (VNS) could help pre-term babies who develop lung infections. "Nearly every one of our chronic diseases, including cancer, Alzheimer's, Parkinson's, chronic arthritis and rheumatoid arthritis, and depression and chronic pain…could benefit from an appropriate stimulator," he said.
It's unfortunate that Kelly got her device only after her large intestine was gone. SetPoint Medical, a privately held California company founded to develop electronic treatments for chronic autoimmune diseases, has announced early positive results with VNS for both Crohn's and rheumatoid arthritis.
As SetPoint's chief medical officer, David Chernoff, put it, "We're hacking into the nervous system to activate a system that is already there," an approach that, he said, could work "on many diseases that are pain- and inflammation-based." Inflammation plays a role in much modern illness, including depression and obesity. The FDA already has approved VNS for both, using surgically implanted devices similar to pacemakers. (GammaCore is external.)
The history of VNS implants goes back to 1997, when the FDA approved one for treating epilepsy and researchers noticed that it rapidly lifted depression in epileptic patients. By 2005, the agency had approved an implant for treatment-resistant depression. (Insurance companies declined to reimburse the approach and it didn't take off, but that might change: in February, the Center for Medicare and Medicaid Services asked for more data to evaluate coverage.) In 2015, the FDA approved an implant in the abdomen to regulate appetite signals and help obese people lose weight.
The link to inflammation had emerged a decade earlier, when researchers at the Feinstein Institute for Medical Research, in Manhasset, New York, demonstrated that stimulating the nerve with electricity in rats suppressed the production of cytokines, a signaling protein important in the immune system. The researchers developed a concept of a hard-wired pathway, through the vagus nerve, between the immune and nervous system. That pathway, they argued, regulates inflammation. While other researchers argue that VNS is helpful by other routes, there is clear evidence that, one way or another, it does affect immunity.
At the same time, investors are seeking alternatives to drugs.
The Feinstein rat research concluded that it took only a minute a day of stimulation and tiny amounts of energy to activate an anti-inflammatory reflex. This means you can use devices "the size of a coffee bean," said Chernoff, much less clunky than current pacemakers—and advances in electronic technology are making them possible.
At the same time, investors are seeking alternatives to drugs. "There's been a push back on drug pricing," noted Lisa Rhoads, a managing director at Easton Capital Investment Group, in New York, which supported electroCore, "and so many unintended consequences."
In 2016, the U.S. National Institutes of Health began pumping money into relevant research, in a program called "Stimulating Peripheral Activity to Relieve Conditions," which focuses on "understanding peripheral nerves — nerves that connect the brain and spinal cord to the rest of the body — and how their electrical signals control internal organ function."
GlaxoSmithKline formed Galvani Bioelectronics with Google to study miniature implants. It had already invested in Action Potential Venture Capital, in Cambridge, Massachusetts, which holds SetPoint and seven other companies "that are all targeting a nerve to treat a chronic disease," noted partner Imran Eba. "I see a future in which bioelectronics medicine is competing directly with drugs," he said.
Treating the body with electricity could bring more ease and lower costs. Many people with serious auto-immune disease, for example, have to inject themselves with drugs that cost $60,000 a year. SetPoint's implant would cost less and only need charging once a week, using a charger worn around the neck, Chernoff said. The company receives notices remotely and can monitor compliance.
Implants also allow the treatment to target a nerve precisely, which could be important with Parkinson's, chronic pain, and depression, observed James Cavuoto, editor and publisher of Neurotech Reports. They may also allow for more fine-turning. "In general, the industry is looking for signals, biomarkers that indicate when is the right time to turn on and turn off the stimulation. It could dramatically increase the effectiveness of the therapy and conserve battery life," he said.
Eventually, external devices could receive data from biomarkers as well. "It could be something you wear on your wrist," Cavuoto noted. Bluetooth-enabled devices could communicate with phones or laptops for data capture. External devices don't require surgery and put the patient in charge. "In the future you'll see more customer specification: Give the patient a tablet or phone app that lets them track and modify their parameters, within a range. With digital devices we have an enormous capability to customize therapies and collect data and get feedback that can be fed back to the clinician," Cavuoto said.
Slow deep breathing, the traditional mind-body intervention, is "like watching Little League. What we're doing is Major League."
It's even possible to stimulate the vagus through the ear, where one branch of the bundle of fibers begins. In a fetus, the tissue that becomes the ear is also part of the vagus nerve, and that one bit remains. "It's the same point as the acupuncture point," explained Mark George, a psychiatrist and pioneer researcher in depression at Medical University of South Carolina in Charleston. "Acupuncture figured out years ago by trial and error what we're just learning about now."
Slow deep breathing, the traditional mind-body intervention, also affects the vagus nerve in positive ways, but gently. "That's like watching Little League," Staats, the co-founder of electroCore, said. "What we're doing is Major League."
In ten years, researcher Wilson suggested, you could be wearing "a little ear cuff" that monitors your basic autonomic tone, a heart-attack risk measure governed in part by the vagus nerve. If your tone looked iffy, the stimulator would intervene, he said, "and improve your mood, cognition, and health."
In the meantime, we can take some long slow breaths, read Whitman, and sing.
“Siri, Read My Mind”: A New Device Lets Users Think Commands
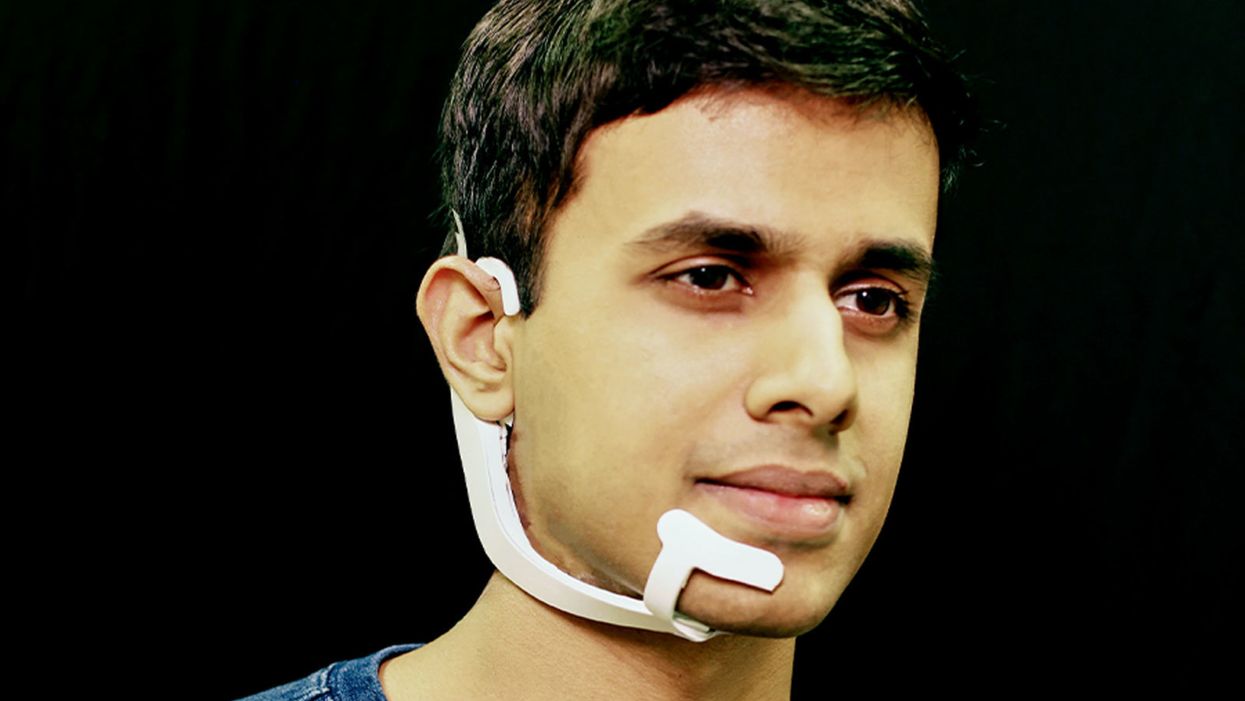
Arnav Kapur, a researcher in the Fluid Interfaces Group at MIT, wears an earlier prototype of the AlterEgo. A newer version is more discreet.
Sometime in the near future, we won't need to type on a smartphone or computer to silently communicate our thoughts to others.
"We're moving as fast as possible to get the technology right, to get the ethics right, to get everything right."
In fact, the devices themselves will quietly understand our intentions and express them to other people. We won't even need to move our mouths.
That "sometime in the near future" is now.
At the recent TED Conference, MIT student and TED Fellow Arnav Kapur was onstage with a colleague doing the first live public demo of his new technology. He was showing how you can communicate with a computer using signals from your brain. The usually cool, erudite audience seemed a little uncomfortable.
"If you look at the history of computing, we've always treated computers as external devices that compute and act on our behalf," Kapur said. "What I want to do is I want to weave computing, AI and Internet as part of us."
His colleague started up a device called AlterEgo. Thin like a sticker, AlterEgo picks up signals in the mouth cavity. It recognizes the intended speech and processes it through the built-in AI. The device then gives feedback to the user directly through bone conduction: It vibrates your inner ear drum and gives you a response meshing with your normal hearing.
Onstage, the assistant quietly thought of a question: "What is the weather in Vancouver?" Seconds later, AlterEgo told him in his ear. "It's 50 degrees and rainy here in Vancouver," the assistant announced.
AlterEgo essentially gives you a built-in Siri.
"We don't have a deadline [to go to market], but we're moving as fast as possible to get the technology right, to get the ethics right, to get everything right," Kapur told me after the talk. "We're developing it both as a general purpose computer interface and [in specific instances] like on the clinical side or even in people's homes."
Nearly-telepathic communication actually makes sense now. About ten years ago, the Apple iPhone replaced the ubiquitous cell phone keyboard with a blank touchscreen. A few years later, Google Glass put computer screens into a simple lens. More recently, Amazon Alexa and Microsoft Cortana have dropped the screen and gone straight for voice control. Now those voices are getting closer to our minds and may even become indistinguishable in the future.
"We knew the voice market was growing, like with getting map locations, and audio is the next frontier of user interfaces," says Dr. Rupal Patel, Founder and CEO of VocalID. The startup literally gives voices to the voiceless, particularly people unable to speak because of illness or other circumstances.
"We start with [our database of] human voices, then train our deep learning technology to learn the pattern of speech… We mix voices together from our voice bank, so it's not just Damon's voice, but three or five voices. They are different enough to blend it into a voice that does not exist today – kind of like a face morph."
The VocalID customer then has a voice as unique as he or she is, mixed together like a Sauvignon blend. It is a surrogate voice for those of us who cannot speak, just as much as AlterEgo is a surrogate companion for our brains.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon."
Voice equality will become increasingly important as Siri, Alexa and voice-based interfaces become the dominant communication method.
It may feel odd to view your voice as a privilege, but as the world becomes more voice-activated, there will be a wider gap between the speakers and the voiceless. Picture going shopping without access to the Internet or trying to eat healthily when your neighborhood is a food desert. And suffering from vocal difficulties is more common than you might think. In fact, according to government statistics, around 7.5 million people in the U.S. have trouble using their voices.
While voice communication appears to be here to stay, at least for now, a more radical shift to mind-controlled communication is not necessarily inevitable. Tech futurist Wagner James Au, for one, is dubious.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon. Generation Z has grown up with smartphones and games like Fortnite, so I don't see them quickly switching to a new form factor. It's still unclear if even head-mounted AR/VR displays will see mass adoption, and mind-reading devices are a far greater physical imposition on the user."
How adopters use the newest brain impulse-reading, voice-altering technology is a much more complicated discussion. This spring, a video showed U.S. House Speaker Nancy Pelosi stammering and slurring her words at a press conference. The problem is that it didn't really happen: the video was manufactured and heavily altered from the original source material.
So-called deepfake videos use computer algorithms to capture the visual and vocal cues of an individual, and then the creator can manipulate it to say whatever it wants. Deepfakes have already created false narratives in the political and media systems – and these are only videos. Newer tech is making the barrier between tech and our brains, if not our entire identity, even thinner.
"Last year," says Patel of VocalID, "we did penetration testing with our voices on banks that use voice control – and our generation 4 system is even tricky for you and me to identify the difference (between real and fake). As a forward-thinking company, we want to prevent risk early on by watermarking voices, creating a detector of false voices, and so on." She adds, "The line will become more blurred over time."
Onstage at TED, Kapur reassured the audience about who would be in the driver's seat. "This is why we designed the system to deliberately record from the peripheral nervous system, which is why the control in all situations resides with the user."
And, like many creators, he quickly shifted back to the possibilities. "What could the implications of something like this be? Imagine perfectly memorizing things, where you perfectly record information that you silently speak, and then hear them later when you want to, internally searching for information, crunching numbers at speeds computers do, silently texting other people."
"The potential," he concluded, "could be far-reaching."