New Options Are Emerging in the Search for Better Birth Control

A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
Dadbot, Wifebot, Friendbot: The Future of Memorializing Avatars
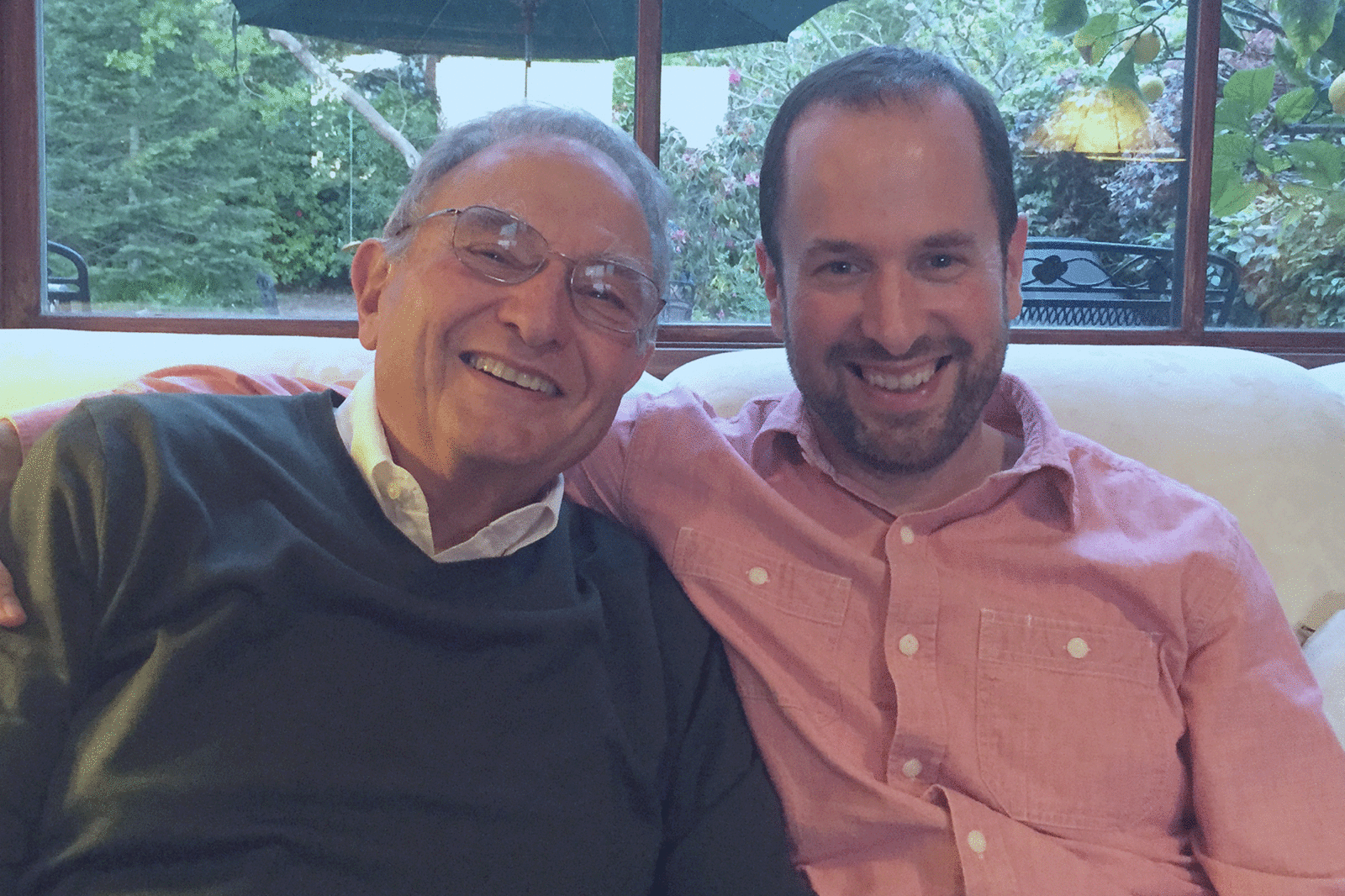
In 2015, about a year before he was diagnosed with terminal lung cancer, John Vlahos posed for a picture with his son, James.
In 2016, when my family found out that my father was dying from cancer, I did something that at the time felt completely obvious: I started building a chatbot replica of him.
I simply wanted to create an interactive way to share key parts of his life story.
I was not under any delusion that the Dadbot, as I soon began calling it, would be a true avatar of him. From my research about the voice computing revolution—Siri, Alexa, the Google Assistant—I knew that fully humanlike AIs, like you see in the movies, were a vast ways from technological reality. Replicating my dad in any real sense was never the goal, anyway; that notion gave me the creeps.
Instead, I simply wanted to create an interactive way to share key parts of his life story: facts about his ancestors in Greece. Memories from growing up. Stories about his hobbies, family life, and career. And I wanted the Dadbot, which sent text messages and audio clips over Facebook Messenger, to remind me of his personality—warm, erudite, and funny. So I programmed it to use his distinctive phrasings; to tell a few of his signature jokes and sing his favorite songs.
While creating the Dadbot, a laborious undertaking that sprawled into 2017, I fixated on two things. The first was getting the programming right, which I did using a conversational agent authoring platform called PullString. The second, far more wrenching concern was my father's health. Failing to improve after chemotherapy and immunotherapy, and steadily losing energy, weight, and the animating sparkle of life, he died on February 9.

John Vlahos at a family reunion in the summer of 2016, a few months after his cancer diagnosis.
(Courtesy James Vlahos)
After a magazine article that I wrote about the Dadbot came out in the summer of 2017, messages poured in from readers. While most people simply expressed sympathy, some conveyed a more urgent message: They wanted their own memorializing chatbots. One man implored me to make a bot for him; he had been diagnosed with cancer and wanted his six-month-old daughter to have a way to remember him. A technology entrepreneur needed advice on replicating what I did for her father, who had stage IV cancer. And a teacher in India asked me to engineer a conversational replica of her son, who had recently been struck and killed by a bus.
Journalists from around the world also got in touch for interviews, and they inevitably came around to the same question. Will virtual immortality, they asked, ever become a business?
The prospect of this happening had never crossed my mind. I was consumed by my father's struggle and my own grief. But the notion has since become head-slappingly obvious. I am not the only person to confront the loss of a loved one; the experience is universal. And I am not alone in craving a way to keep memories alive. Of course people like the ones who wrote me will get Dadbots, Mombots, and Childbots of their own. If a moonlighting writer like me can create a minimum viable product, then a company employing actual computer scientists could do much more.
But this prospect raises unanswered and unsettling questions. For businesses, profit, and not some deeply personal mission, will be the motivation. This shift will raise issues that I didn't have to confront. To make money, a virtual immortality company could follow the lucrative but controversial business model that has worked so well for Google and Facebook. To wit, a company could provide the memorializing chatbot for free and then find ways to monetize the attention and data of whoever communicated with it. Given the copious amount of personal information flowing back and forth in conversations with replica bots, this would be a data gold mine for the company—and a massive privacy risk for users.
Virtual immortality as commercial product will doubtless become more sophisticated.
Alternately, a company could charge for memorializing avatars, perhaps with an annual subscription fee. This would put the business in a powerful position. Imagine the fee getting hiked each year. A customer like me would find himself facing a terrible decision—grit my teeth and keep paying, or be forced to pull the plug on the best, closest reminder of a loved one that I have. The same person would effectively wind up dying twice.
Another way that a beloved digital avatar could die is if the company that creates it ceases to exist. This is no mere academic concern for me: Earlier this year, PullString was swallowed up by Apple. I'm still able to access the Dadbot on my own computer, fortunately, but the acquisition means that other friends and family members can no longer chat with him remotely.
Startups like PullString, of course, are characterized by impermanence; they tend to get snapped up by bigger companies or run out of venture capital and fold. But even if big players like, say, Facebook or Google get into the virtual immortality game, we can't count on them existing even a few decades from now, which means that the avatars enabled by their technology would die, too.
The permanence problem is the biggest hurdle faced by the fledgling enterprise of virtual immortality. So some entrepreneurs are attempting to enable avatars whose existence isn't reliant upon any one company or set of computer servers. "By leveraging the power of blockchain and decentralized software to replicate information, we help users create avatars that live on forever," says Alex Roy, the founder and CEO of the startup Everlife.ai. But until this type of solution exists, give props to conventional technology for preserving memories: printed photos and words on paper can last for centuries.
The fidelity of avatars—just how lifelike they are—also raises serious concerns. Before I started creating the Dadbot, I worried that the tech might be just good enough to remind my family of the man it emulated, but so far off from my real father that it gave us all the creeps. But because the Dadbot was a simple chatbot and not some all-knowing AI, and because the interface was a messaging app, there was no danger of him encroaching on the reality of my actual dad.
But virtual immortality as commercial product will doubtless become more sophisticated. Avatars will have brains built by teams of computer scientists employing the latest techniques in conversational AI. The replicas will not just text but also speak, using synthetic voices that emulate the ones of the people being memorialized. They may even come to life as animated clones on computer screens or in 3D with the help of virtual reality headsets.
What fascinates me is how technology can help to preserve the past—genuine facts and memories from peoples' lives.
These are all lines that I don't personally want to cross; replicating my dad was never the goal. I also never aspired to have some synthetic version of him that continued to exist in the present, capable of acquiring knowledge about the world or my life and of reacting to it in real time.
Instead, what fascinates me is how technology can help to preserve the past—genuine facts and memories from people's lives—and their actual voices so that their stories can be shared interactively after they have gone. I'm working on ideas for doing this via voice computing platforms like Alexa and Assistant, and while I don't have all of the answers yet, I'm excited to figure out what might be possible.
[Adapted from Talk to Me: How Voice Computing Will Transform the Way We Live, Work, and Think (Houghton Mifflin Harcourt, March 26, 2019).]
The Best Kept Secret on the International Space Station
Rendering of Space Tango ST-42 autonomous commercial manufacturing platform in Low Earth Orbit. (Courtesy Space Tango)
[Editor's Note: This video is the second of a five-part series titled "The Future Is Now: The Revolutionary Power of Stem Cell Research." Produced in partnership with the Regenerative Medicine Foundation, and filmed at the annual 2019 World Stem Cell Summit, this series illustrates how stem cell research will profoundly impact life on earth.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.