New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
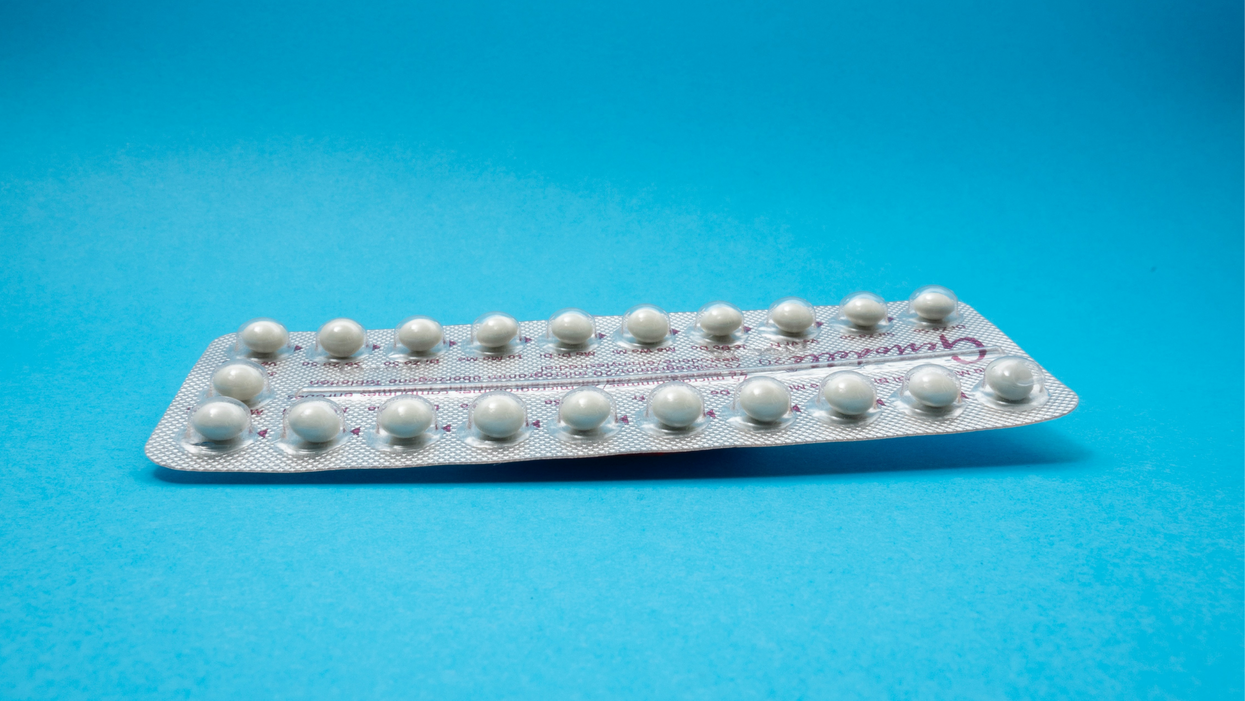
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
New Cell Therapies Give Hope to Diabetes Patients
Researchers are developing new cell therapies that could cure Type 1 diabetes and make constant management of the disease a way of the past.
For nearly four decades, George Huntley has thought constantly about his diabetes. Diagnosed in 1983 with Type 1 (insulin-dependent) diabetes, Huntley began managing his condition with daily finger sticks to check his blood glucose levels and doses of insulin that he injected into his abdomen. Even now, with an insulin pump and a device that continuously monitors his glucose, he must consider how every meal will affect his blood sugar, checking his monitor multiple times each hour.
Like many of those who depend on insulin injections, Huntley is simultaneously grateful for the technology that makes his condition easier to manage and tired of thinking about diabetes. If he could wave a magic wand, he says, he would make his diabetes disappear. So when he read about biotechs like ViaCyte and Vertex Pharmaceuticals developing new cell therapies that have the potential to cure Type 1 diabetes, Huntley was excited.
You also won’t see him signing up any time soon. The therapies under development by both companies would require a lifelong regimen of drugs for suppressing the immune system to prevent the body from rejecting the foreign cells. It’s a problem also seen in the transplant of insulin-producing cells of the pancreas – called islet cells – from deceased donors. To Howard Foyt, chief medical officer at ViaCyte, a San Diego-based biotech specializing in the development of cell therapies for diabetes, the tradeoff is worth it.
“A lot of the symptoms of diabetes are not something that you wear on your arm, so to speak. You’re not necessarily conscious of them until you’re successfully treated, and you feel better,” Foyt says.
For many with diabetes, managing these symptoms is a constant game of Whack-a-Mole. “Any form of treatment that gets someone closer to feeling good is a victory,” he says.
“Am I going to be trading diabetes for cancer? That’s not a chance I
want to take."
But not everyone is convinced. What’s more, it’s likely that the availability of these cell therapies will be limited to those with life-threatening diabetes symptoms, such as hypoglycemia unawareness. To Huntley, these therapies remain a bit of a Faustian bargain.
“Am I going to be trading diabetes for cancer? That’s not a chance I want to take,” he says.
The discovery of insulin in 1921 transformed Type 1 diabetes from a death sentence into a potentially manageable condition. Even as better versions of insulin hit the market—ones that weren’t derived from pigs and wouldn’t provoke an allergic response, longer-acting insulin, insulin pens—they didn’t change the reality that those with Type 1 diabetes remained dependent on insulin. Even the most advanced continuous glucose monitors (which tests blood sugar levels every few minutes, 24/7) and insulin pumps don’t perform as well as a healthy pancreas.
Whether by injection or pump, someone with diabetes needs to administer the insulin their body no longer makes. With advances in organ transplantation, the concept of transplanting insulin-producing pancreatic beta cells seemed obvious. After more than a decade of painstaking work, James Shapiro, who directs the Islet Transplant Program at the University of Albania, honed a process called the Edmonton Protocol for pancreas transplants. For a few patients who couldn’t control their blood sugars any other way, the Edmonton Protocol became a life saver. Some of these patients were even able to stop insulin completely, Shapiro says. But the high cost of organ transplant and a chronic shortage of donor organs, pancreas or otherwise, meant that only a small handful of patients could benefit.
Stem cells, however, can be grown in vats, meaning that supply would never be an issue. “We would be going from a very successful treatment of today to a potential cure tomorrow,” Shapiro says.
In 2014, spurred by his own children’s diagnoses with Type 1 Diabetes, stem cell biologist Doug Melton of Harvard University figured out a way to differentiate embryonic stem cells into functional pancreatic beta cells. It was a long process, explains immunoengineer Alice Tomei at the University of Miami, because “the islet is not one cell, it's like a mini-organ that has its own needs.”
Add on the risk of rejection and autoimmunity, and Tomei says that scientists soon realized that chronic and systemic immunosuppression was the only way forward. Over the next several years, Melton improved his approach to yield more cells with fewer impurities. Melton partnered with Boston-based Vertex Pharmaceuticals to create a cell therapy called VX-880.
The first patient received his dose earlier in 2021. In October, Vertex released 90-day results from the Phase 1/2 trial, which revealed the patient was able to reduce his insulin usage from an average of 34 units per day to just 2.9 units per day. The tradeoff is a lifelong need for immunosuppressive drugs to prevent the body from attacking both foreign cells and pancreatic beta cells. It’s what recipients of ViaCyte’s first-gen PEC-Direct will also need. For Foyt, it’s an easy choice.
“At this point in time, immunosuppression is the necessary evil,” he says. “For parents, would you like to worry about going into your child’s bedroom every morning and not knowing if they’re going to be alive or dead? It’s uncommon, but it does occur.”
Not everyone, however, finds the trade-off easy to swallow. Especially with COVID-19 cases reaching record highs, the prospect of reducing his immune function at a time when he needs it most doesn’t sit well with Huntley. The risks of immunosuppression also mean that diabetes cell therapies are limited to those patients with life-threatening complications.
It’s why ViaCyte has created two new iterations of cellular therapies that would eliminate this need. The ViaCyte-Encap contains the cells in a permeable container that allows oxygen, insulin, and nutrients to flow freely but prevents immune system access. Their latest model, PEC-QT, just began safety trials with Shapiro’s lab at the University of Alberta and uses gene editing to eliminate any cellular markers that would trigger an immune response.
Sanjoy Dutta, vice president of research at JDRF International, a nonprofit that funds the study of diabetes, is thrilled with the progress that’s been made around cell therapies, but he cautions it’s still early days. “We have proven that these cells can be made. What we haven’t seen is are they going to work for six months, two years, five years? It’s a challenge we still need to overcome,” he says.
Iowa social worker Jodi Lynn’s concerns echo Dutta’s. Lynn was diagnosed with diabetes in 1998 at age 14 after a bout of severe influenza, spends each day inventorying supplies, planning her food intake, and maintaining her insulin pump and glucose monitor. These newer technologies dramatically improved her blood sugar control but, like everyone with diabetes, Lynn remains at high risk for complications, such as diabetic ketoacidosis, heart disease, vision loss, and kidney failure. Lynn, already considered immunocompromised due to medications she takes for another autoimmune condition, is less concerned with immune suppression than the untested nature of these therapies.
“I want to know that they will work long-term,” she says.
How Genetic Testing and Targeted Treatments Are Helping More Cancer Patients Survive
Scientists are studying cancer genomes to more precisely diagnose their patients' diseases - offering hope for targeted drug treatments.
Late in 2018, Chris Reiner found himself “chasing a persistent cough” to figure out a cause. He talked to doctors; he endured various tests, including an X-ray. Initially, his physician suspected bronchitis. After several months, he still felt no improvement. In May 2019, his general practitioner recommended that Reiner, a business development specialist for a Seattle-based software company, schedule a CAT scan.
Reiner knew immediately that his doctor asking him to visit his office to discuss the results wasn’t a good sign. The longtime resident of Newburyport, MA, remembers dreading “that conversation that people who learn they have cancer have.”
“The doctor handed me something to look at, and the only thing I remember after that was everything went blank all around me,” Reiner, 50, reveals. “It was the magnitude of what he was telling me, that I had a malignant mass in my lung.”
Next, he recalls, he felt ushered into “the jaws of the medical system very quickly.” He spent a couple of days meeting with a team of doctors at Beth Israel Deaconess Medical Center in nearby Boston. One of them was from a medical field he hadn’t even known existed, a pulmonary interventionist, who would perform a biopsy on the mass in his lung.
“Knowing there was a medicine for my particular type of cancer was like a weight lifted off my shoulders."
A week later he and his wife Allison returned to meet with the oncologist, radiologist, pulmonary interventionist – his medical team. They confirmed his initial diagnosis: Stage 4 metastatic lung cancer that had spread to several parts of his body. “We just sat there, stunned,” he says. “I felt like I was getting hit by a wrecking ball over and over.”
An onslaught of medical terminology about what they had identified flowed over the shocked couple, but then the medical team switched gears, he recalls. They offered hope. “They told me, ‘Hey, you’re not a smoker, so that’s good,’” Reiner says. “‘There’s a good chance that what’s driving this disease for you is actually a genetic mutation, and we have ways to understand more about what that could be through some simple testing.’”
They told him about Foundation Medicine, a company launched in neighboring Cambridge, MA, in 2009 that develops, manufactures, and sells genomic profiling assays. These are tests that, according to the company’s website, “can analyze a broad panel of genes to detect the four main classes of genomic alterations known to drive cancer growth.” With these insights, certain patients can be matched with therapies targeted specifically for the genetic driver(s) of their cancer. The company maintains one of the largest cancer genomic databases in the world, with more than 500,000 patient samples profiled, and they have more than 65 biopharma partners.
According to Foundation Medicine, they are the only company that has FDA-approved tests for both tissue- and blood-based comprehensive genomic profiling tests. One other company has an FDA-approved biopsy test, and several other companies offer tissue-based genomic profiling. Additionally, several major cancer centers like Memorial Sloan Kettering in New York and Anderson Cancer Center in Texas have their own such testing platforms.
Currently, genomic profiling is more accessible for patients with advanced cancer, due to broader insurance coverage in later stages of disease.
“Right now, the vast majority of patients either have cancers for which we don’t have treatments or they have genetic alterations that are not known,” says Jorge Garcia, MD, Division Chief, Solid Tumor Oncology, UH Cleveland Medical Center, which has its own CGP testing platform. “However, a significant proportion of patients with advanced cancer have alterations that we can tap for therapeutic purposes.”
Foundation Medicine estimates that in 2017, just over 5 percent of advanced solid cancer patients in the U.S. received CGP testing. In 2021, they estimate that number is between 25 to 30 percent of advanced solid cancer patients in the U.S., which doesn’t include patients who are tested with small (less than 50 genes) panels. Their panel tests for more than 300 cancer-related genes.

“The good news is the platforms we are developing are better and more comprehensive, and they’re going to continue to be larger data sets,” Dr. Garcia adds.
In Reiner’s case, his team ordered comprehensive genetic profiling on both his tissue and blood, from Foundation Medicine.
At this point, Reiner still wasn’t sure what genetic mutations were or how they factored into cancer or what comprehensive genomic profiling entailed. That day, though, his team ushered the Reiners into the world of precision oncology that placed him on much more sure footing to learn about and fight the specific lung cancer that had been troubling him for more than a year.
What genetic alterations were driving his cancer? Foundation Medicine’s tests were about to find out.
At the core of these tests is next generation sequencing, a DNA sequencing technology. Since 2009, this has revolutionized genomic research, according to the National Center for Biotechnology Information, because it allows an entire human genome to be sequenced within one day. Cancer genomics posits that cancer is caused by mutations and is a disease of the genome. Now, cancer genomes can be systemically studied in their entirety. For cancer patients such as Reiner, NGS can provide a more precise diagnosis and classification of the disease, more accurate prognosis, and potentially the identification of targeted drug treatments. Ultimately, the technology can provide the basis of personalized cancer management.
The detailed reports supply patients and their oncologists with extensive information about the patient’s genomic profile and potential treatment options that they can discuss together. Reiner trusted his doctors that this approach was worth the two- or three-week wait to receive the Foundation Medicine report and the specifically targeted treatment, rather than immediately jump into a round of chemotherapy. He is especially grateful now, he says, because the report delivered a great deal of relief from his previously exhausting and growing anxiety about having cancer.
Reiner and his team learned his lung cancer contained the epidermal growth factor receptor (EGFR) mutation. That biomarker enabled his oncologist to prescribe Tagrisso (osimertinib), a medication developed to directly target that genetic mutation.
“Knowing there was a medicine for my particular type of cancer was like a weight lifted off my shoulders,” he says. “It only took a week or two before my cough finally started subsiding. This pill goes right after the particular piece of genetic material in the tumor that’s causing its growth.”
Dr. Jerry Mitchell, director field medical oncology, Foundation Medicine, in Columbus, Ohio, explains that genomic profiling is generating substantial impacts today. “This is a technology that is the standard of care across many advanced malignancies that takes patients from chemotherapy-only options to very targeted options or immunotherapy options,” he says. “You can also look at complex biomarkers, and these are not specific genetic changes but different genes across the tumor to get a biomarker.”
According to Dr. Mitchell, Foundation Medicine’s technology can test more than 324 different cancer-related genes in a single test. Thus, a growing number of patients are benefitting from comprehensive genetic profiling, due to the rapidly growing number of targeted therapies. While not all of the cancers are treatable yet, the company uses that information to partner with researchers to find new potential therapies for patient groups that may have rare mutations.
Since his tumor’s diagnosis, Reiner has undergone chemotherapy and a couple surgeries to treat the metastatic cancer in other parts of his body, but the drug Tagrisso has significantly reduced his lung tumor. Now, having learned so much during the past couple of years, he is grateful for precision oncology. He still reflects on the probability that, had the Tagrisso pill not been available in May 2019, he might have only survived for another six months or a year.
“Comprehensive Genomic Profiling is not some future state, but in both the U.S. and Europe, it is a very standard, accepted, and recommended first step to knowing how to treat your cancer,” says Dr. Mitchell, adding that he feels fortunate to be an oncologist in this era. “However, we know there are still people not getting this recommended testing, so we still have opportunities to find many more patients and impact them by knowing the molecular profile of their cancer.”

