New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
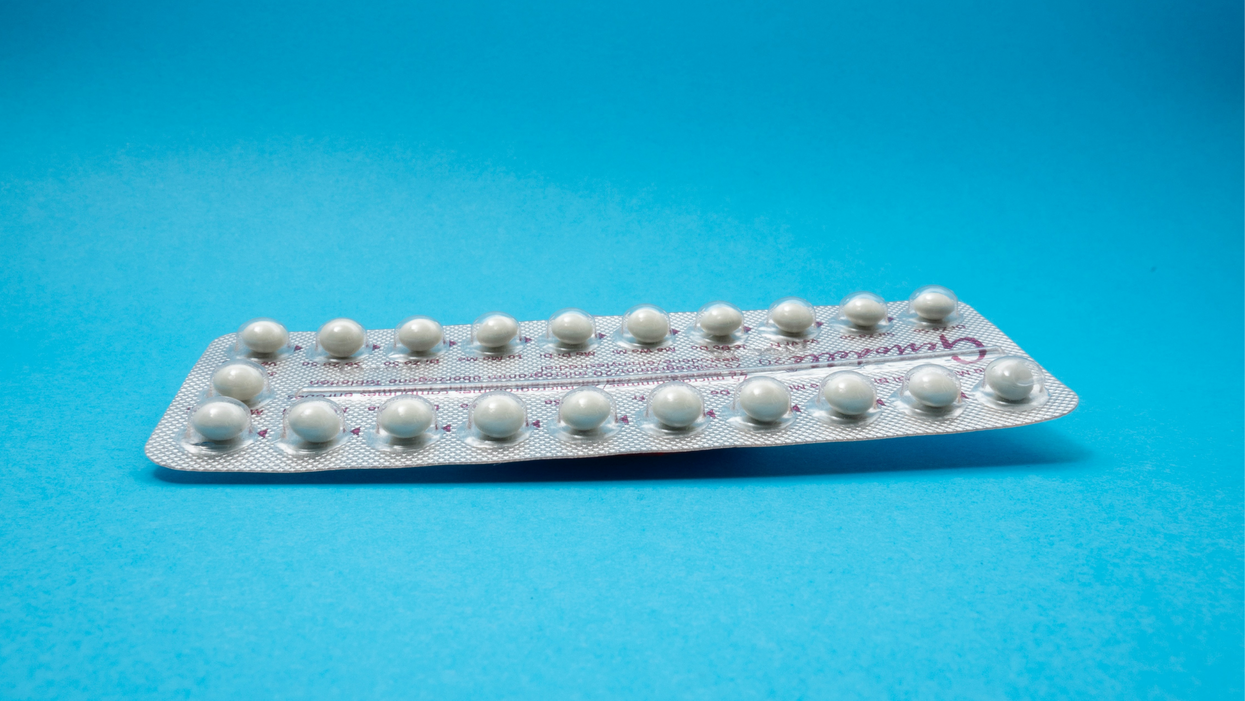
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
The Pandemic Is Ushering in a More Modern—and Ethical—Way of Studying New Drugs and Diseases
Miniature lab-grown models of human organs have major advantages over standard animal testing.
Before the onset of the coronavirus pandemic, Dutch doctoral researcher Joep Beumer had used miniature lab-grown organs to study the human intestine as part of his PhD thesis. When lockdown hit, however, he was forced to delay his plans for graduation. Overwhelmed by a sense of boredom after the closure of his lab at the Hubrecht Institute, in the Netherlands, he began reading literature related to COVID-19.
"By February [2020], there were already reports on coronavirus symptoms in the intestinal tract," Beumer says, adding that this piqued his interest. He wondered if he could use his miniature models – called organoids -- to study how the coronavirus infects the intestines.
But he wasn't the only one to follow this train of thought. In the year since the pandemic began, many researchers have been using organoids to study how the coronavirus infects human cells, and find potential treatments. Beumer's pivot represents a remarkable and fast-emerging paradigm shift in how drugs and diseases will be studied in the coming decades. With future pandemics likely to be more frequent and deadlier, such a shift is necessary to reduce the average clinical development time of 5.9 years for antiviral agents.
Part of that shift means developing models that replicate human biology in the lab. Animal models, which are the current standard in biomedical research, fail to do so—96% of drugs that pass animal testing, for example, fail to make it to market. Injecting potentially toxic drugs into living creatures, before eventually slaughtering them, also raises ethical concerns for some. Organoids, on the other hand, respond to infectious diseases, or potential treatments, in a way that is relevant to humans, in addition to being slaughter-free.

Human intestinal organoids infected with SARS-CoV-2 (white).
Credit: Joep Beumer/Clevers group/Hubrecht Institute
Urgency Sparked Momentum
Though brain organoids were previously used to study the Zika virus during the 2015-16 epidemic, it wasn't until COVID-19 that the field really started to change. "The organoid field has advanced a lot in the last year. The speed at which it happened is crazy," says Shuibing Chen, an associate professor at Weill Cornell Medicine in New York. She adds that many federal and private funding agencies have now seen the benefits of organoids, and are starting to appreciate their potential in the biomedical field.
Last summer, the Organo-Strat (OS) network—a German network that uses human organoid models to study COVID-19's effects—received 3.2 million euros in funding from the German government. "When the pandemic started, we became aware that we didn't have the right models to immediately investigate the effects of the virus," says Andreas Hocke, professor of infectious diseases at the Charité Universitätsmedizin in Berlin, Germany, and coordinator of the OS network. Hocke explained that while the World Health Organization's animal models showed an "overlap of symptoms'' with humans, there was "no clear reflection" of the same disease.
"The network functions as a way of connecting organoid experts with infectious disease experts across Germany," Hocke continues. "Having organoid models on demand means we can understand how a virus infects human cells from the first moment it's isolated." Overall, OS aims to create infrastructure that could be applied to future pandemics. There are 28 sub-projects involved in the network, covering a wide assortment of individual organoids.
Cost, however, remains an obstacle to scaling up, says Chen. She says there is also a limit to what we can learn from organoids, given that they only represent a single organ. "We can add drugs to organoids to see how the cells respond, but these tests don't tell us anything about drug metabolism, for example," she explains.
A Related "Leaps" in Progress
One way to solve this issue is to use an organ-on-a-chip system. These are miniature chips containing a variety of human cells, as well as small channels along which functions like blood or air flow can be recreated. This allows scientists to perform more complex experiments, like studying drug metabolism, while producing results that are relevant to humans.

An organ-on-a-chip system.
Credit: Fraunhofer IGB
Such systems are also able to elicit an immune response. The FDA has even entered into an agreement with Wyss Institute spinoff Emulate to use their lung-on-a-chip system to test COVID-19 vaccines. Representing multiple organs in one system is also possible. Berlin-based TissUse are aiming to make a so-called 'human on a chip' system commercially available. But TissUse senior scientist Ilka Maschmeyer warns that there is a limit to how far the technology can go. "The system will not think or feel, so it wouldn't be possible to test for illnesses affecting these abilities," she says.
Some challenges also remain in the usability of organs-on-a-chip. "Specialized training is required to use them as they are so complex," says Peter Loskill, assistant professor and head of the organ-on-a-chip group at the University of Tübingen, Germany. Hocke agrees with this. "Cell culture scientists would easily understand how to use organoids in a lab, but when using a chip, you need additional biotechnology knowledge," he says.
One major advantage of both technologies is the possibility of personalized medicine: Cells can be taken from a patient and put onto a chip, for example, to test their individual response to a treatment. Loskill also says there are other uses outside of the biomedical field, such as cosmetic and chemical testing.
"Although these technologies offer a lot of possibilities, they need time to develop," Loskill continues. He stresses, however, that it's not just the technology that needs to change. "There's a lot of conservative thinking in biomedical research that says this is how we've always done things. To really study human biology means approaching research questions in a completely new way."
Even so, he thinks that the pandemic marked a shift in people's thinking—no one cared how the results were found, as long as it was done quickly. But Loskill adds that it's important to balance promise, potential, and expectations when it comes to these new models. "Maybe in 15 years' time we will have a limited number of animal models in comparison to now, but the timescale depends on many factors," he says.
Beumer, now a post-doc, was eventually allowed to return to the lab to develop his coronavirus model, and found working on it to be an eye-opening experience. He saw first-hand how his research could have an impact on something that was affecting the entire human race, as well as the pressure that comes with studying potential treatments. Though he doesn't see a future for himself in infectious diseases, he hopes to stick with organoids. "I've now gotten really excited about the prospect of using organoids for drug discovery," he says.
The coronavirus pandemic has slowed society down in many respects, but it has flung biomedical research into the future—from mRNA vaccines to healthcare models based on human biology. It may be difficult to fully eradicate animal models, but over the coming years, organoids and organs-on-a-chip may become the standard for the sake of efficacy -- and ethics.
Jack McGovan is a freelance science writer based in Berlin. His main interests center around sustainability, food, and the multitude of ways in which the human world intersects with animal life. Find him on Twitter @jack_mcgovan."
New Podcast: Why Dr. Ashish Jha Expects a Good Summer
Dr. Jha discusses Covid vaccine passports, how supply and demand of the vaccines is about to shift, the AstraZeneca situation, what's new with kids, herd immunity, and more.
Making Sense of Science features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
Hear the 30-second trailer:
Listen to the whole episode: "Why Dr. Ashish Jha Expects a Good Summer"
Dr. Ashish Jha, dean of public health at Brown University, discusses the latest developments around the Covid-19 vaccines, including supply and demand, herd immunity, kids, vaccine passports, and why he expects the summer to look very good.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.