New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
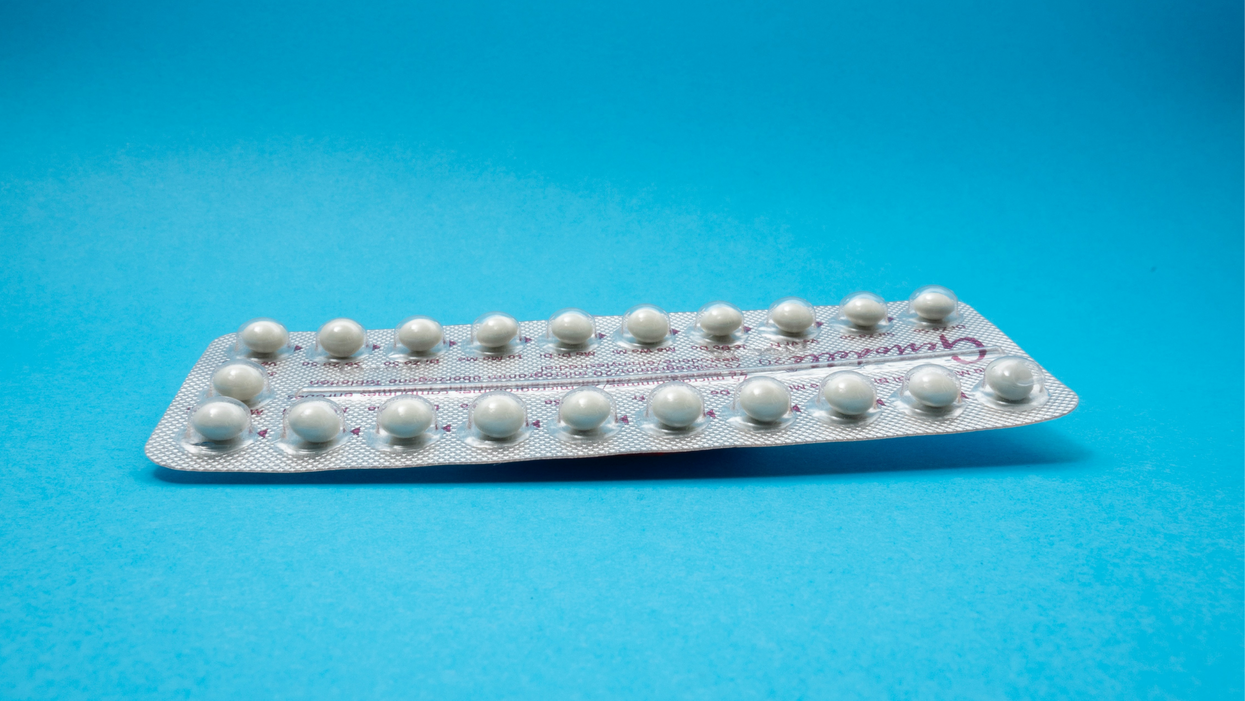
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
A newly discovered brain cell may lead to better treatments for cognitive disorders
Swiss researchers have found a type of brain cell that appears to be a hybrid of the two other main types — and it could lead to new treatments for brain disorders.
Swiss researchers have discovered a third type of brain cell that appears to be a hybrid of the two other primary types — and it could lead to new treatments for many brain disorders.
The challenge: Most of the cells in the brain are either neurons or glial cells. While neurons use electrical and chemical signals to send messages to one another across small gaps called synapses, glial cells exist to support and protect neurons.
Astrocytes are a type of glial cell found near synapses. This close proximity to the place where brain signals are sent and received has led researchers to suspect that astrocytes might play an active role in the transmission of information inside the brain — a.k.a. “neurotransmission” — but no one has been able to prove the theory.
A new brain cell: Researchers at the Wyss Center for Bio and Neuroengineering and the University of Lausanne believe they’ve definitively proven that some astrocytes do actively participate in neurotransmission, making them a sort of hybrid of neurons and glial cells.
According to the researchers, this third type of brain cell, which they call a “glutamatergic astrocyte,” could offer a way to treat Alzheimer’s, Parkinson’s, and other disorders of the nervous system.
“Its discovery opens up immense research prospects,” said study co-director Andrea Volterra.
The study: Neurotransmission starts with a neuron releasing a chemical called a neurotransmitter, so the first thing the researchers did in their study was look at whether astrocytes can release the main neurotransmitter used by neurons: glutamate.
By analyzing astrocytes taken from the brains of mice, they discovered that certain astrocytes in the brain’s hippocampus did include the “molecular machinery” needed to excrete glutamate. They found evidence of the same machinery when they looked at datasets of human glial cells.
Finally, to demonstrate that these hybrid cells are actually playing a role in brain signaling, the researchers suppressed their ability to secrete glutamate in the brains of mice. This caused the rodents to experience memory problems.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Andrea Volterra, University of Lausanne.
But why? The researchers aren’t sure why the brain needs glutamatergic astrocytes when it already has neurons, but Volterra suspects the hybrid brain cells may help with the distribution of signals — a single astrocyte can be in contact with thousands of synapses.
“Often, we have neuronal information that needs to spread to larger ensembles, and neurons are not very good for the coordination of this,” researcher Ludovic Telley told New Scientist.
Looking ahead: More research is needed to see how the new brain cell functions in people, but the discovery that it plays a role in memory in mice suggests it might be a worthwhile target for Alzheimer’s disease treatments.
The researchers also found evidence during their study that the cell might play a role in brain circuits linked to seizures and voluntary movements, meaning it’s also a new lead in the hunt for better epilepsy and Parkinson’s treatments.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Volterra.
Researchers claimed they built a breakthrough superconductor. Social media shot it down almost instantly.
In July, South Korean scientists posted a paper finding they had achieved superconductivity - a claim that was debunked within days.
Harsh Mathur was a graduate physics student at Yale University in late 1989 when faculty announced they had failed to replicate claims made by scientists at the University of Utah and the University of Wolverhampton in England.
Such work is routine. Replicating or attempting to replicate the contraptions, calculations and conclusions crafted by colleagues is foundational to the scientific method. But in this instance, Yale’s findings were reported globally.
“I had a ringside view, and it was crazy,” recalls Mathur, now a professor of physics at Case Western Reserve University in Ohio.
Yale’s findings drew so much attention because initial experiments by Stanley Pons of Utah and Martin Fleischmann of Wolverhampton led to a startling claim: They were able to fuse atoms at room temperature – a scientific El Dorado known as “cold fusion.”
Nuclear fusion powers the stars in the universe. However, star cores must be at least 23.4 million degrees Fahrenheit and under extraordinary pressure to achieve fusion. Pons and Fleischmann claimed they had created an almost limitless source of power achievable at any temperature.
Like fusion, superconductivity can only be achieved in mostly impractical circumstances.
But about six months after they made their startling announcement, the pair’s findings were discredited by researchers at Yale and the California Institute of Technology. It was one of the first instances of a major scientific debunking covered by mass media.
Some scholars say the media attention for cold fusion stemmed partly from a dazzling announcement made three years prior in 1986: Scientists had created the first “superconductor” – material that could transmit electrical current with little or no resistance. It drew global headlines – and whetted the public’s appetite for announcements of scientific breakthroughs that could cause economic transformations.
But like fusion, superconductivity can only be achieved in mostly impractical circumstances: It must operate either at temperatures of at least negative 100 degrees Fahrenheit, or under pressures of around 150,000 pounds per square inch. Superconductivity that functions in closer to a normal environment would cut energy costs dramatically while also opening infinite possibilities for computing, space travel and other applications.
In July, a group of South Korean scientists posted material claiming they had created an iron crystalline substance called LK-99 that could achieve superconductivity at slightly above room temperature and at ambient pressure. The group partners with the Quantum Energy Research Centre, a privately-held enterprise in Seoul, and their claims drew global headlines.
Their work was also debunked. But in the age of internet and social media, the process was compressed from half-a-year into days. And it did not require researchers at world-class universities.
One of the most compelling critiques came from Derrick VanGennep. Although he works in finance, he holds a Ph.D. in physics and held a postdoctoral position at Harvard. The South Korean researchers had posted a video of a nugget of LK-99 in what they claimed was the throes of the Meissner effect – an expulsion of the substance’s magnetic field that would cause it to levitate above a magnet. Unless Hollywood magic is involved, only superconducting material can hover in this manner.
That claim made VanGennep skeptical, particularly since LK-99’s levitation appeared unenthusiastic at best. In fact, a corner of the material still adhered to the magnet near its center. He thought the video demonstrated ferromagnetism – two magnets repulsing one another. He mixed powdered graphite with super glue, stuck iron filings to its surface and mimicked the behavior of LK-99 in his own video, which was posted alongside the researchers’ video.
VanGennep believes the boldness of the South Korean claim was what led to him and others in the scientific community questioning it so quickly.
“The swift replication attempts stemmed from the combination of the extreme claim, the fact that the synthesis for this material is very straightforward and fast, and the amount of attention that this story was getting on social media,” he says.
But practicing scientists were suspicious of the data as well. Michael Norman, director of the Argonne Quantum Institute at the Argonne National Laboratory just outside of Chicago, had doubts immediately.
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication.
“It wasn’t a very polished paper,” Norman says of the Korean scientists’ work. That opinion was reinforced, he adds, when it turned out the paper had been posted online by one of the researchers prior to seeking publication in a peer-reviewed journal. Although Norman and Mathur say that is routine with scientific research these days, Norman notes it was posted by one of the junior researchers over the doubts of two more senior scientists on the project.
Norman also raises doubts about the data reported. Among other issues, he observes that the samples created by the South Korean researchers contained traces of copper sulfide that could inadvertently amplify findings of conductivity.
The lack of the Meissner effect also caught Mathur’s attention. “Ferromagnets tend to be unstable when they levitate,” he says, adding that the video “just made me feel unconvinced. And it made me feel like they hadn't made a very good case for themselves.”
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication. Despite being debunked, cold fusion claimants Pons and Fleischmann didn’t disappear. They moved their research to automaker Toyota’s IMRA laboratory in France, which along with the Japanese government spent tens of millions of dollars on their work before finally pulling the plug in 1998.
Fusion has since been created in laboratories, but being unable to reproduce the density of a star’s core would require excruciatingly high temperatures to achieve – about 160 million degrees Fahrenheit. A recently released Government Accountability Office report concludes practical fusion likely remains at least decades away.
However, like Pons and Fleischman, the South Korean researchers are not going anywhere. They claim that LK-99’s Meissner effect is being obscured by the fact the substance is both ferromagnetic and diamagnetic. They have filed for a patent in their country. But for now, those claims remain chimerical.
In the meantime, the consensus as to when a room temperature superconductor will be achieved is mixed. VenGennep – who studied the issue during his graduate and postgraduate work – puts the chance of creating such a superconductor by 2050 at perhaps 50-50. Mathur believes it could happen sooner, but adds that research on the topic has been going on for nearly a century, and that it has seen many plateaus.
“There's always this possibility that there's going to be something out there that we're going to discover unexpectedly,” Norman notes. The only certainty in this age of social media is that it will be put through the rigors of replication instantly.