New Podcast: "Making Sense of Science"
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Making Sense of Science features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
Episode 1: "COVID-19 Vaccines and Our Progress Toward Normalcy"
Bioethicist Arthur Caplan of NYU shares his thoughts on when we will build herd immunity, how enthusiastic to be about the J&J vaccine, predictions for vaccine mandates in the coming months, what should happen with kids and schools, whether you can hug your grandparents after they get vaccinated, and more.
Transcript:
KIRA: Hi, and welcome to our new podcast 'Making Sense of Science', the show that features interviews with leading experts in health and science about the latest developments and the big ethical questions. I'm your host Kira Peikoff, the editor of leaps.org. And today, we're going to talk about the Covid-19 vaccines. I'm honored that my first guest is Dr. Art Caplan of NYU, one of the world's leading bio-ethicists. Art, thanks so much for joining us today.
DR. CAPLAN: Thank you so much for having me.
KIRA: So the big topic right now is the new J&J vaccine, which is likely to be given to millions of Americans in the coming weeks. It only requires one-shot, it can be stored in refrigerators for several months. It has fewer side-effects and most importantly, it is extremely effective at the big things, preventing hospitalizations and deaths. Though not as effective as Pfizer and Moderna in preventing moderate cases, especially potentially in older adults with underlying conditions. So Art, what's your take overall, on how enthusiastic Americans should be about this vaccine?
DR. CAPLAN: I'm usually enthusiastic. The more weapons, the better. This vaccine, while maybe, slightly less efficacious than the Moderna and the Pfizer ones, is easier to make, is easier to ship. It's one-shot. You know, here there's already been problems of getting people to come back in for their second shots. I would say 5... 7% of people don't show up even though you remind them and you nag them, they don't come back. So a one-shot option is great. A one-shot option that's easy to, if you will, brew up in your rural pharmacy without having to have special instructions is great. And I think it's gonna really facilitate herd-immunity, meaning, we'll see millions and millions and millions of doses of the Janssen vaccine out there as an option, I'm gonna say, summer.
KIRA: Great. And to be fair, it's worth mentioning that the J&J vaccine was tested in clinical trials after variants began to circulate, and it's only one-shot instead of two, like the other vaccines, and it gets more effective over time. So is it really fair to directly compare its efficacy to the mRNA vaccines?
DR. CAPLAN: Well, you know, people are gonna do that. And one issue that'll come up ethically is people are gonna say, "Can I choose my vaccine? I want the most efficacious one. I want the name brand that I trust. I don't want the new platform. I like Janssen's 'cause it's an older, more established way to make vaccines or whatever." Who knows what cuckoo-cockamamie reasons they might have. To me, you take what you can get, it'll be great. It's way above what we normally would expect, those 95% success rates are off the charts. Getting something that's 70% effective, it's perfectly wonderful. I wish we had flu shots that were 70% effective.
And the other thing to keep in mind is we're gonna see more mutations, we're gonna see more strains. That's just a reality of viruses. So they'll mutate, more strains will appear, we can't just say, "Oh my goodness. There's a South-African one or the California one or the UK one. We better... I don't know, do something different." We're just gonna have to basically resign ourselves, I think, to boosters. So right now, take the vaccine. I'm almost tempted to say, "Shut up and take the vaccine. Don't worry about choosing."
Just get what you can get. If you live in a rural community and all they have is Janssen, take it. If you're in another country and all they ship to you is Janssen, take it. And then we'll worry about the next round of virus mutations, if you will, when we get to the boosters. I'm more concerned that these things aren't gonna last more than a year or two than I am that they're not gonna pick up every mutation.
KIRA: So on that note, shipping to rural places or low-income countries that lack the ultra-cold freezers that you need for the super effective mRNA vaccines, the Janssen vaccine seems like a really great option, but are we going to encounter a potential conflict of people saying, "Well, there's "poor or rich vaccines," and one is slightly less effective than the other." And so are we gonna disenfranchise people and undermine their actual willingness to take the vaccine?
DR. CAPLAN: Well, it's interesting. I think the first problem is gonna be, "I have vaccine and I don't have any vaccine," between rich and poor countries. Look, the poor countries are screaming to get vaccine supply sent to them. I think, for example, Ghana received recently 600 million doses of AstraZeneca vaccine. It was freed up by South Africa, which decided they didn't wanna use it 'cause they thought there was "a better vaccine" coming. So even among the poorer nations or the developing nations, some vaccines are getting typed as the not-as-good or the less-desirable... We've already started to see it.
But for the most part, the rich countries are gonna try and vaccinate to herd immunity, you can argue about the ethics of whether that's right, before they start sharing. And I think we'll have haves and have-nots, herd immunity produced in the rich countries, Japan, North America, Europe, by the end of the year anyway. And still some countries floundering around saying, "I didn't get anything," and what are you gonna do?
KIRA: And I know you said to people, which is a very memorable quote, "just shut up and take the vaccine, whatever you can get, whatever is available to you now, do it." But inevitably, as you mentioned, some people are going to say, "Well, I just wanna wait to get the best one possible." When will people have a choice in vaccines, do you think?
DR. CAPLAN: I don't think you'll see that till next year. I think we're gonna see distribution according to where the supply chains are that the vaccine manufacturers use. So if I use McKesson and they ship to the Northeast, and that's where my vaccine goes, that's what's available there. If I'm contracted to Walmart and they buy Janssen, that's what you're gonna see at the big box store. I don't think you're really gonna get too much in the way of choice until next year, when then they're gonna say they ship three different kinds of vaccine, and I can offer you one dose or two dose... One of them lasts a year, one of them lasts 18 months. I don't think we're gonna have the informed choice until next year.
KIRA: Okay. And right now the steep demand is outstripping the supply, and there's been a lot of pressure put on the vaccine makers to ramp up as quickly as possible. Of course, they say that they're doing that and the government is pressuring them to do that, but when do you think we'll cross over to the point where vaccine hesitancy is a bigger issue than vaccine demand?
DR. CAPLAN: Yeah. So this is a really interesting issue. I'm glad you asked me this because I think it's got good foresight. The big ethics fight now is scarcity and who goes first, and the ethicists, including me, are having a fine old time arguing about healthcare workers versus policemen versus people who work for UPS versus somebody who's working at the drug store. Who's more important? Why are they more important? Who's essential?
Actually, I think most of that is nonsense, because what we've learned is that you can't do much in the way of micro-allocation, the system strains, and it doesn't work. You've gotta use some pretty broad categories like over 65, still breathing and working, and a kid. The kid will go last, 'cause we don't have the data, everybody else should get in line and the over 65s should probably be first 'cause they're at high risk. We can't do this. We stink at the micro-management of vaccine supply, plus it encourages cheating. So everybody's out there with vaccine hunters, vaccine tourism, bribing, lying, dressing up like a grandmother to get a vaccine. My favorite one was some rich people in Vancouver flew up to the Yukon and pretended to be Inuit aboriginal people to get a vaccine. That will all pass.
We'll have enough vaccine by the summer, more or less, that the issue will then be, "How are we gonna get to herd immunity or at least maximal immunity, knowing that we don't have data on kids?" People under 18, I think are something like 20% of our population. That means the best you could do is 80%. The other population still could be passing the virus, kids here or Europe or wherever. Well, the military refusal rate that I just saw was 30% saying no. I've heard nursing home staff rates, nursing attendants, nursing aids up at 40% to 50% saying no. So these are huge refusal rates, people are nervous about how it works, the vaccine. Some of them are like, "Well Art you take it. If you're still alive in six months, then maybe I'll take it, but I wanna see that it really works and it's safe." And other people say, "We don't wanna be exploited. We don't trust the government, whatever, to offer us these vaccines."
I'm gonna answer that was a long-winded way of saying we're gonna see some mandates, we're gonna see some coercions start to show up in the vaccine supply, because I think, for example, the military. The day one of these license gets... Excuse me, one of these vaccines get licensed, right now they're on an emergency approval, collect data for three or four more months, get the FDA to formally license the thing. I'd say between five minutes and 10 minutes, the military will be mandating. They have no interest in your objection, they have no interest in your choice, they know what the mission is. It's traditionally, we're gonna get you as healthy as we can to fight a war.
The fact that you say, "Gee, I might die." They kind of say, "Yeah, we noticed that, but that's in the military culture. We fight wars and do stuff like that." So they'll be mandating, I think, very rapidly. And I think healthcare workers will. I think most hospitals are gonna say 50% refusal rate among this nursing group? Forget it. We can't risk that. Nursing homes have been devastated by COVID. They're not gonna have aids out there unvaccinated. The only thing holding up the mandates right now is that we don't have full licensure. We have emergency use approvals, and that's good.
But it's a little tough to mandate without full license. The day we get it, three months, four months, we're gonna start to see mandates. And I'll make one more prediction, as long as I'm in a crystal ball mode. It won't be the government at that point that says, you have to be vaccinated. It'll be private business, 'cause they're gonna say, "You know what? Come on my cruise ship, 'cause everybody who works here is vaccinated." "Come on into my bar, everybody who works here is vaccinated." They're gonna start to use it as an advertising marketing lure. "It's safe here. Come on in." So I think they'll say, "If you wanna work on an airline as a flight attendant, you get vaccinated. We have vaccine proof. You can show it on your iPhone, on your whatever, you have a card that you did it." And so I think we'll see many businesses moving to vaccinate so that they can bring their customers back in.
KIRA: So private businesses, that's one thing, because people do not have to patronize those places if they don't wanna get vaccinated. But of course, this is gonna open up a can of worms with schools. Public schools, if they mandate teacher vaccines and you have to send your kid to school and you have to go to work at a school. What happens then?
DR. CAPLAN: Well, schools are gonna be at the end of the line. That's where we have the least data. So I don't think we're gonna see school mandates on kids, maybe not till next year. But we already have school mandates on kids. They were the first group to feel the force of mandates, because it turns out that measles and mumps and whooping cough are easy to get at school, sneezing and coughing on one another. Some states have added flu shots. Many states, California, Maine, New York have actually eliminated exemptions. The only way out for those kids is if they have a health reason. They're not even allowing religious or so-called philosophical or personal choice exemptions. COVID vaccines will just line up right next to those things.
Teachers will demand it, the pressure will be there. We'll have a lot of information by next year on safety. I'm even gonna say people are gonna be less tolerant of non-vaccinators. Now it's sort of like, "Wow. Yeah, I guess." But this time next year, if you haven't vaccinated, people are gonna come to your house and board it up and make you stay inside.
KIRA: Well, given how much we're so dependent on these vaccines to get us back to a regular life, I can understand the sentiment. What is your take on the big controversy right now, just going back towards the present day a little bit more on having kids in schools. Is that something you support before all the teachers have been vaccinated?
DR. CAPLAN: I do, but I have a problem with the definition of a teacher and a school. So by the way, some people that I know, friends of mine have said, "Well, I'm a teacher, I'm a yoga instructor. I'm a teacher, I'm an aerobics instructor. So I should get priority access to vaccination." I don't think that's what we meant by teacher. And here's the difference in schools. I live in Ridgefield, Connecticut. Up the street for me is a very fancy private elementary school. It has endless grounds, open classrooms. If there are eight kids in a class, I'll pass out. It is great. I wish I went to college there. It's a wonderful set up. Do they need to vaccinate everybody? Probably not, they're all sitting six feet apart, everybody in there is gonna mask, they have huge auditoriums. They never have to come in contact.
I've been in some other schools in the Bronx. No ventilation, no plumbing, 35 kids in a class, the teacher's 65. And you sort of think, "Boy, I'd wanna have vaccinate everybody in sight in this place because unless we re-haul the buildings and downsize the class size, people are gonna get sick in here."
They probably were getting sick anyway before COVID, but now COVID makes it worse. They're probably getting the flu or colds at nine times the rate that they were in Ridgefield, Connecticut. So my point is this, high school kids doing certain things, they can come in on a mixed schedule three days a week, two days a week, do their thing, they know how to mask. Am I worried about vaccinating there? Not too much. Elementary school kids need psychosocial development, need to learn social skills, sometimes going to schools that aren't that wonderful. Yeah, let's vaccinate them. So even though I was complaining a bit about micro-management and trying to parse out, here I think you need to do it. I think you're probably gonna say college, I don't know that you have to vaccinate there. High schools, 50/50. Elementary school, let's do them first.
KIRA: Got it. And one more question on kids before I wanna move on, there's been talk about whether it's necessary before kids are allowed to get this vaccine to have the FDA go to full approval with the full bulk of data necessary for that versus just an emergency authorization for the general population, given that kids are at so much lower risk than adults. But then of course, it'll take a lot more time, I imagine, to get the kids the vaccines. What's your take on that?
DR. CAPLAN: We historically have demanded higher levels of evidence to do anything with a kid, and I think that's gonna hold true here too. I don't think you're gonna see emergency-use authorization for people under 18. Maybe they'd cut it and say, "We'll do it 12-18," but just looking at the history of drug development, vaccine development, people are really leery of taking risks with kids and appropriately so. Kids can't even make their own decision. I can decide if I wanna take an emergency-use vaccine, if I think it's too iffy I don't take it right now. So up to me to weigh the risk-benefit. I don't think so. I think you'll see licensing required before we really get it, at least 12 and under. Let's put it there. And I'm not worried about the safety or efficacy of these things in kids. I think there's no reason, given the biological mechanisms, to think they're gonna be any different. But it's gonna be pretty tough pre-licensure to impose anything.
KIRA: And when do you think that licensure for kids under 12 could come?
DR. CAPLAN: Well, two groups of people are now being studied, pregnant women, the studies just launched. They'll probably be done sufficiently by the end of the year. Kids for full licensure, spring next year.
KIRA: Okay. And because this is a big question for a lot of women that I know and women in general who are pregnant, what would you say to them now, where we don't have the data yet on the safety, but they have to decide and they can't wait six or nine more months?
DR. CAPLAN: Vaccinate yesterday. Literally, I think the COVID virus is too dangerous, I think it's dangerous to the mom, I think it's dangerous to the fetus. It is an unknown, but boy, I would bet on the vaccine more than I would taking my chances with the virus.
KIRA: Got it. So let's pivot a little bit and talk about some of the big open questions around the vaccines that we're starting to get some early evidence about. For one thing, do they prevent transmission and not just symptomatic disease. And I think it's worth pointing out for our audience here that there is a big difference between preventing symptoms and preventing infections, as lots of asymptomatic people know. And we have a lot of new real-world evidence from Israel, from Scotland, reporting that even asymptomatic infections are greatly reduced by the Pfizer vaccine, for example. What is your take on how this new data is going to change guidance around post-vaccination behaviors?
DR. CAPLAN: Yeah. What do we got in the podcast? Seven or eight hours to go? That's a tough one. It's complicated. But trying to over-simplify a little bit. So there is a difference, and this has gotten confusing, I fear. Some vaccines prevent you from getting infected at all. It looks like the Pfizer and the Moderna fall into that category. That's great, 'cause no matter what else, it probably means you're gonna reduce transmission, 'cause if you can't be infected, I don't know how you're gonna give it to somebody else. So I'll bet that that's a transmission reduction. Looks like Johnson & Johnson, unclear. Seems to prevent bad symptoms and death but not moderate disease, and it isn't clear that it stops you from getting infected. So that may become an issue in terms of how we strategically approach when we have enough vaccine of the different types. We may wanna say, "Look, in some environments, we've gotta control spread... Nursing home. We wanna see the Moderna there. We wanna see the Pfizer there."
In other situations, we just wanna make sure you're not dead, let's get the Janssen thing out there. And that'll be great. I'll tell you... I'll give you an example from my own current existence. So I've been pretty cautious... As I said, I live in Ridgefield, Connecticut. I have a house, pretty roomy, but I haven't left it very often. I'm willing to take the chance to go shopping. I'll confess I'm even willing to take the chance wearing a mask to go to the drug store and I've had a hair-cut or two. So I've been not hyper-cautious, but cautious. I don't invite people over that I don't know where they've been, so to speak. But now I'm vaccinated, and my wife is fully vaccinated. And the other night for the first time, we went out to an indoor restaurant. Probably haven't done that in 10 months... No, I don't know, six months. But a long time...
KIRA: I hope you really enjoyed that first meal out, 'cause that's something that I dream about. Boy, where am I gonna go and what am I gonna order?
DR. CAPLAN: Yeah. We went to the fanciest restaurant in town, as a matter of fact, and they were social distancing and everybody was masked and the wait staff. But I figure, good enough for me. If the thing isn't gonna kill me, if I was just told I was gonna have a risk of being sick for three days or something, that's good enough for me. I don't wanna infect somebody else. So I'll still mask and do that, I'm not sure. But I'm absolutely ready to say, and in fact, I've scheduled two trips. We're gonna take a trip to Florida, we're gonna take a trip to North Carolina in March and April. I'm figuring even then, things will be better. But everybody's gonna have choices like that to make. It'll be really interesting. If I'm Tony Fauci or one of our big public health guys, I don't want anybody going anywhere, I'm risk-averse, until maybe 2027. I think it'll be controlled and eliminated... We'll have lots of data and everything will be great. I'm a little bit more, shall we say, individual choice-oriented, making individual risk things, like I said. As long as I'm responsible to others.
I don't wanna make anybody else sick, but if I am ready to take the chance of just being sick for a few days, and I believe the vaccines available will keep me out of the hospital and keep me out of the Morticians building. Okay, I'm ready to do it. So each one of us is gonna have to make a value decision, this is what I find interesting, about what's normal. It isn't science. It isn't medicine. It's ethics. You're gonna have to decide how much risk do you wanna take. Do you wanna be a jerk to your neighbor, if you could still have a teeny chance of infecting them? Am I willing to live in a world where COVID is around but it's kinda rare? I know kids are still transmitting, but it's not really a huge risk. That's the kind of value choice that each of us will be faced with.
KIRA: I really appreciate your emphasis on individual choice and values here and letting... Basically allowing people to make those judgments based on their circumstances for themselves. If you're not deathly afraid of getting a mild cold-type illness, then I can understand why you wanna fly or go to a restaurant, and other people might not be comfortable with any risk at all, and they're perfectly welcome to stay home.
DR. CAPLAN: Or they may say, "I'm 80, I have nine chronic diseases. A mild illness still freaks me out." Okay, I get that. I'm perfectly respectful of that. It's interesting. I think we've been used to public health messaging, and people have this attitude that at some point, Fauci or the head of the CDC, somebody's gonna show up on TV and say, "All clear, everything's over, back to normal, we've declared victory over the enemy. It's armistice day." Whatever. It isn't gonna work like that is my prediction. It's gonna be a slow creep, different people deciding, "I'm safe enough, I'm wandering out." Other people say, "No, no, not ready." Or somebody saying, "I'm pregnant. I'm staying in. I don't care what's going on. I'm not gonna take that risk." I think people will be surprised that there isn't going to be a national day of resolution or something. [chuckle]
KIRA: Right. It's more about these individual behaviors and over time, letting people decide what to do. So for example, if you had grandkids and they were not vaccinated, but you are, would you hug them, would you get close to them, how would you behave and how do you think they should behave around you?
DR. CAPLAN: So I'd be still nervous about them transmitting, but I'm also a very strong believer in my vaccine. So yes, I would hug them, and yes, I would have them come to visit. And that's probably gonna happen actually fairly soon. But their parents aren't vaccinated yet. And so I'm still nervous that maybe better not to do a lot of social mingling right now. But yeah, people have said to me, "My grandmom is 94. I don't know how long she's gonna be here. You think if I'm vaccinated it's okay to pay a visit." I'm gonna start to say, "Yeah, I get that."
KIRA: And I think one thing that's lost in these discussions of safety is also the aspect of benefits to human life and why we even live in the first place. We don't live lives of complete safety. We drive, we fly, we do things that are risky, but we take those risks, because it's worth it. So I think that should be part of the discussion overall, not just safety, period.
DR. CAPLAN: And not just saving lives. So ski slopes, there are a lot of orthopedic clinics at the bottom of big ski slopes, and sending a message like, "You can break bones here." But people say, "I wanna do it, I enjoy it." Okay, I'm not sure all the time that we should factor all of that into our pooled insurance plan, but that's a fight for another day. Nonetheless, I would... You know something, I would pay for it 'cause I like to encourage people to enjoy themselves. So I have my bad habits, they have their bad habits. I think it's sort of a wash in a certain way. But more to your point, I think if you look out there and say, there are some areas where we don't let you choose. You must put your kid in a car seat. A kid can't make a decision, the thing is very effective, really saves their lives, they should have a life ahead of them, and we're gonna force it. And I'm all for that.
In other instances, I might go into the restaurant. I think it's part of the general, "Am I gonna drive a car, am I gonna cross a busy street... " As you said, there are many things I have to do where I have to think about the risk-benefit. I may make a lousy calculation and underestimate what it means to get in my car and drive in terms of risk relative to getting hit by lightening or some other risks, but that's a little bit more for me.
KIRA: So that's a really thought-provoking conversation, but I wanna switch for a minute to another question mark around the vaccines besides transmission, is the long-term studies of their effects on the immune system. And one thing that I've noticed some experts are concerned about is the fact that a lot of the people in the placebo groups have dropped out of the trials and gotten the vaccine because ethically you can't withhold the vaccinations from these volunteers, but at the same time, that could be hurting our ability to compare the vaccine's long-term effects against people who haven't had the vaccine for a long time. So how significant is this issue in your mind?
DR. CAPLAN: Big. Some people actually proposed that we not let them drop out, we not tell the subjects in these big trials of vaccines if they were in the placebo group. Can't do that. It's clearly unethical... Achieved consensus on that decades ago, with various studies where the researcher said, "We don't have to tell the subjects that there's a treatment." Tuskegee did that, for example, the horrible study in the early, late '60s, early '70s, where they didn't tell people there was a cure and kept the study going of venereal disease, but there have been many others since. We already know you gotta give them the option. Some people may stay in anyway, but not enough to allow the study to really have integrity. So I think current studies are likely to fall apart and we won't get answers in the way we're used to with randomized trials to the long-term effects or even to the how long does it last question.
We need to build a system that can follow people. We can't rely on them being in an observed clinical trial. We have to start to say, "You register, we're gonna check on you every year to see how you're doing." That's gotta be done. And one other provocative idea, I pushed it long ago, challenge studies. Deliberately infect a small group of people, hopefully healthy people that choose to do it with mild COVID and then see what the vaccine does in them and then get an answer faster if you study them over time, they volunteered knowingly to get exposed this way. I think you're gonna see some challenge studies done particularly to compare vaccines. There are still more vaccines coming, maybe some of them will last longer, cheaper, safer, I don't know. The only way you're gonna study the next round of vaccines is in a challenge study. You're never getting anybody to sign up to be in a placebo control randomized trial.
KIRA: So that was actually my next question, that the UK just approved the first ever challenge study to infect the volunteers on purpose with the virus. Now, the UK has often been much more progressive in doing medical research than the US. Do you think the US will ever get to that point or are we just gonna rely on other countries to do that for us?
DR. CAPLAN: I think we won't get there. We're so conservative, so litigation conscious. People are freaked out that if somebody got sick and died in a challenge study, it would bankrupt the sponsor. I think the UK is on the right path, but I don't really think we're gonna follow.
KIRA: Okay, well, I hope that they can do the work that we really need. And I'm grateful that there are other countries that are more permissive of risk-taking and doing the controversial studies that are required.
DR. CAPLAN: Ironically, if you don't do the challenge studies, the only other way you're gonna get to do big-scale randomized placebo trials is in the poorest countries that can't get anything. And that makes it an awful lot like exploitation, taking advantage, as opposed to choice. But that's where you'd go, you'd say, "Oh, I got this new vaccine, I'll test it out in Sierra Leone and they don't have anything anyway. So better that half of them get the vaccine than not." And I still think the challenge study makes more ethic sense.
KIRA: Yeah, absolutely. That would really be a shame to be put in that position instead of just allowing people to decide. We let people sign up for the army where they might die. What's the ethical difference with signing up for a potentially dangerous study, but if you're young and healthy, the risk is low?
DR. CAPLAN: By the way, the risk from COVID to say, 18 to 35-year-olds, who's who you'd be looking at, is about the same as donating a kidney, which we also allow all the time.
KIRA: Right, right. Great point. Before we finish up here, I just wanna quickly touch on, of course, the big elephant in the room, which we all have to deal with, unfortunately, which is the variants. So I wanna talk about where we stand. I've heard some vaccine experts recently say, like Paul Offit, for example, has said he doesn't expect a fourth surge due to this, but others are more cautious and take the flip side saying, "This is the calm before the storm. We're about to see another huge explosion." California has recently reported a new strain as accounts for maybe potentially 50% of cases now, and it could be 90% by the end of March. But we're seeing such big declines in the numbers in hospitalizations, in cases. So what should people make of these conflicting messages?
DR. CAPLAN: There's an attitude in medicine that many doctors take toward things like incipient or new prostate cancer, sometimes toward breast cancer, or at least lumps. It's called watchful waiting. You pay attention. You watch what's going on. But you don't do anything right away. I would still get vaccinated, I would still take what I could get. I still believe that it's likely that these vaccines are gonna provide some protection, if not against infection, then at least against the worst symptoms and the worst chances of dying because they're really gonna boost up the basic immune system, which should be able to start to fight against viruses.
That said, could we wind up with some virulent new strain that evades the current vaccine platforms? Yes. Is it likely? I don't think so. But what it does mean is get ready to get boosters because the response to new strains that have been a result of viral mutations is you gotta adjust your vaccine. That's what we'll do. I hope it doesn't send us back into quarantine and isolation and distancing and all the rest of it as our only control. I'm hoping that the manufacturers can roll out boosters more quickly than the first round of vaccines.
KIRA: And the FDA has just said that the vaccine developers will not need to start over with new clinical trials to these boosters. So that will greatly expedite the process. And do you think that's the right call?
DR. CAPLAN: Yes, absolutely. You're not changing the fundamental nature of the vaccine platform, you're just tweaking, if you will, which chemistry responds to the virus. So yeah, I do.
KIRA: And one question then that necessarily everyone is gonna wonder is, "Well, if I got the J&J vaccine, can I get an mRNA booster?" Can you mix and match? Is that gonna work for your immune system?
DR. CAPLAN: Yeah. We don't have any idea. And I wouldn't do that right away. I know some countries are thinking about that to get more, if you will, use out of a limited supply. I'd say wait three months and do it the right way, where the data is in evidence. I'm not worried about people getting a second shot of something different and dropping dead. I'm just worried that it won't work. [chuckle] So I'm not a fan of mix and match. You can do it in some studies, by the way. You could do it in some challenge studies and get a faster answer than you would having to try and do this in 30,000 people over a year. But no, I don't think that's a good way to go. And I'm not a big fan of one-shot strategies either. I think, what we know is that the second shot really kicks your immune system into high gear and that's what you want for real protection. So I know why people say it but I wouldn't advocate for it.
KIRA: Right. And for my last question. One of our big themes this year that we'll be following all throughout the year at leaps.org is our progress towards an eventual return to life and return to normalcy. So I have to ask that question to you. Given everything that you know and that we've discussed today, when do you think our lives and society will start to look normal again, with schools, and restaurants, and businesses open, people are flying and gathering without fear, traveling, etcetera?
DR. CAPLAN: I think you're gonna see a lot of that this summer. There's gonna be enough vaccine out there, even if the epidemiologists aren't 100% happy. As I said, I think a lot of people are gonna say, "I'm happy enough, good enough for me. I'm going to sports and I'm flying, and I'm taking a vacation." And we'll be outside again. Remember we had the ability to eat outdoors and congregate less when the weather's better around the whole country, and I think that will open up Europe and the US in addition. What I'm worried about is if we had to go back in the fall to a more controlled environment, either 'cause a new strain appeared, or just because things weren't as efficacious as we hoped they'd be. But I think summer is gonna be good this year.
KIRA: Well, I hope you're right. I hope your crystal ball is working today. [chuckle]
DR. CAPLAN: [chuckle] And if it's not working right, email Kira. Don't talk to me.
KIRA: Yeah, I cannot be held liable for this. Thank you Art for a fascinating discussion. And thanks to everyone for listening. If you like this show, follow Making Sense of Science to hear new episodes coming once a month. And if you wanna give us feedback, we'd love to hear from you. Get in touch on our website, leaps.org. And until next time, thanks everyone.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
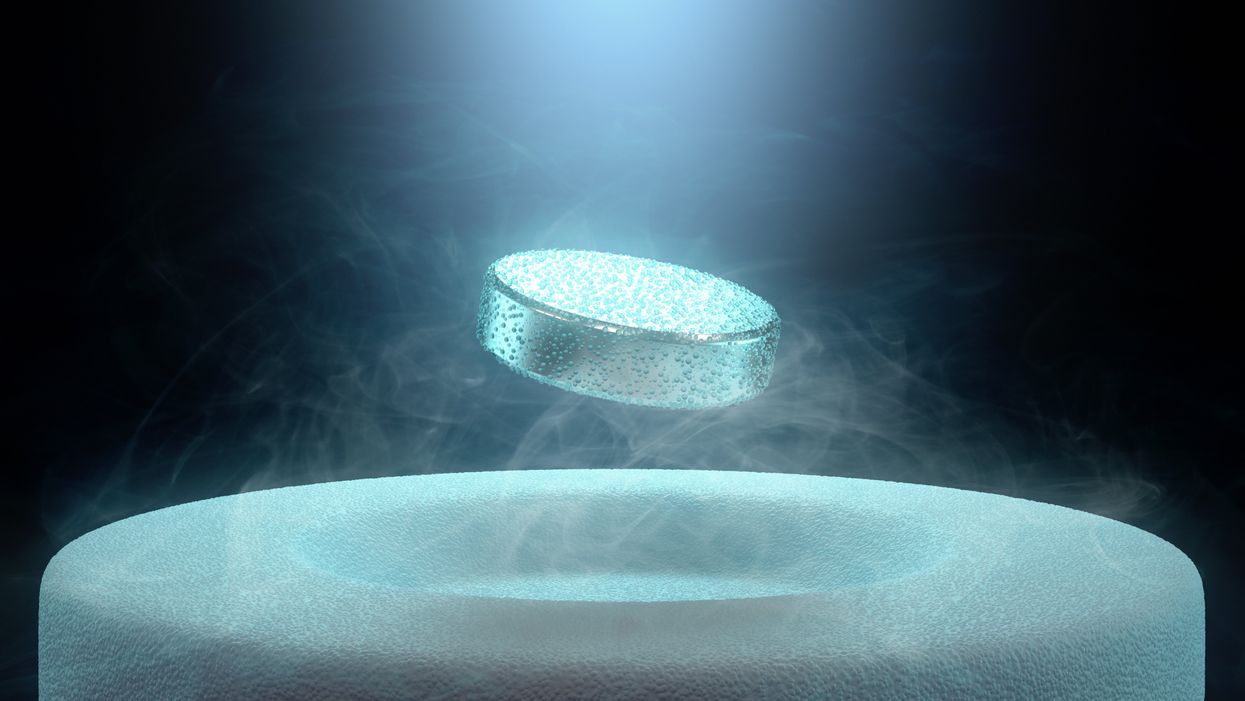
Researchers claimed they built a breakthrough superconductor. Social media shot it down almost instantly.
In July, South Korean scientists posted a paper finding they had achieved superconductivity - a claim that was debunked within days.
Harsh Mathur was a graduate physics student at Yale University in late 1989 when faculty announced they had failed to replicate claims made by scientists at the University of Utah and the University of Wolverhampton in England.
Such work is routine. Replicating or attempting to replicate the contraptions, calculations and conclusions crafted by colleagues is foundational to the scientific method. But in this instance, Yale’s findings were reported globally.
“I had a ringside view, and it was crazy,” recalls Mathur, now a professor of physics at Case Western Reserve University in Ohio.
Yale’s findings drew so much attention because initial experiments by Stanley Pons of Utah and Martin Fleischmann of Wolverhampton led to a startling claim: They were able to fuse atoms at room temperature – a scientific El Dorado known as “cold fusion.”
Nuclear fusion powers the stars in the universe. However, star cores must be at least 23.4 million degrees Fahrenheit and under extraordinary pressure to achieve fusion. Pons and Fleischmann claimed they had created an almost limitless source of power achievable at any temperature.
Like fusion, superconductivity can only be achieved in mostly impractical circumstances.
But about six months after they made their startling announcement, the pair’s findings were discredited by researchers at Yale and the California Institute of Technology. It was one of the first instances of a major scientific debunking covered by mass media.
Some scholars say the media attention for cold fusion stemmed partly from a dazzling announcement made three years prior in 1986: Scientists had created the first “superconductor” – material that could transmit electrical current with little or no resistance. It drew global headlines – and whetted the public’s appetite for announcements of scientific breakthroughs that could cause economic transformations.
But like fusion, superconductivity can only be achieved in mostly impractical circumstances: It must operate either at temperatures of at least negative 100 degrees Fahrenheit, or under pressures of around 150,000 pounds per square inch. Superconductivity that functions in closer to a normal environment would cut energy costs dramatically while also opening infinite possibilities for computing, space travel and other applications.
In July, a group of South Korean scientists posted material claiming they had created an iron crystalline substance called LK-99 that could achieve superconductivity at slightly above room temperature and at ambient pressure. The group partners with the Quantum Energy Research Centre, a privately-held enterprise in Seoul, and their claims drew global headlines.
Their work was also debunked. But in the age of internet and social media, the process was compressed from half-a-year into days. And it did not require researchers at world-class universities.
One of the most compelling critiques came from Derrick VanGennep. Although he works in finance, he holds a Ph.D. in physics and held a postdoctoral position at Harvard. The South Korean researchers had posted a video of a nugget of LK-99 in what they claimed was the throes of the Meissner effect – an expulsion of the substance’s magnetic field that would cause it to levitate above a magnet. Unless Hollywood magic is involved, only superconducting material can hover in this manner.
That claim made VanGennep skeptical, particularly since LK-99’s levitation appeared unenthusiastic at best. In fact, a corner of the material still adhered to the magnet near its center. He thought the video demonstrated ferromagnetism – two magnets repulsing one another. He mixed powdered graphite with super glue, stuck iron filings to its surface and mimicked the behavior of LK-99 in his own video, which was posted alongside the researchers’ video.
VanGennep believes the boldness of the South Korean claim was what led to him and others in the scientific community questioning it so quickly.
“The swift replication attempts stemmed from the combination of the extreme claim, the fact that the synthesis for this material is very straightforward and fast, and the amount of attention that this story was getting on social media,” he says.
But practicing scientists were suspicious of the data as well. Michael Norman, director of the Argonne Quantum Institute at the Argonne National Laboratory just outside of Chicago, had doubts immediately.
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication.
“It wasn’t a very polished paper,” Norman says of the Korean scientists’ work. That opinion was reinforced, he adds, when it turned out the paper had been posted online by one of the researchers prior to seeking publication in a peer-reviewed journal. Although Norman and Mathur say that is routine with scientific research these days, Norman notes it was posted by one of the junior researchers over the doubts of two more senior scientists on the project.
Norman also raises doubts about the data reported. Among other issues, he observes that the samples created by the South Korean researchers contained traces of copper sulfide that could inadvertently amplify findings of conductivity.
The lack of the Meissner effect also caught Mathur’s attention. “Ferromagnets tend to be unstable when they levitate,” he says, adding that the video “just made me feel unconvinced. And it made me feel like they hadn't made a very good case for themselves.”
Will this saga hurt or even affect the careers of the South Korean researchers? Possibly not, if the previous fusion example is any indication. Despite being debunked, cold fusion claimants Pons and Fleischmann didn’t disappear. They moved their research to automaker Toyota’s IMRA laboratory in France, which along with the Japanese government spent tens of millions of dollars on their work before finally pulling the plug in 1998.
Fusion has since been created in laboratories, but being unable to reproduce the density of a star’s core would require excruciatingly high temperatures to achieve – about 160 million degrees Fahrenheit. A recently released Government Accountability Office report concludes practical fusion likely remains at least decades away.
However, like Pons and Fleischman, the South Korean researchers are not going anywhere. They claim that LK-99’s Meissner effect is being obscured by the fact the substance is both ferromagnetic and diamagnetic. They have filed for a patent in their country. But for now, those claims remain chimerical.
In the meantime, the consensus as to when a room temperature superconductor will be achieved is mixed. VenGennep – who studied the issue during his graduate and postgraduate work – puts the chance of creating such a superconductor by 2050 at perhaps 50-50. Mathur believes it could happen sooner, but adds that research on the topic has been going on for nearly a century, and that it has seen many plateaus.
“There's always this possibility that there's going to be something out there that we're going to discover unexpectedly,” Norman notes. The only certainty in this age of social media is that it will be put through the rigors of replication instantly.
Scientists implant brain cells to counter Parkinson's disease
In a recent research trial, patients with Parkinson's disease reported that their symptoms had improved after stem cells were implanted into their brains. Martin Taylor, far right, was diagnosed at age 32.
Martin Taylor was only 32 when he was diagnosed with Parkinson's, a disease that causes tremors, stiff muscles and slow physical movement - symptoms that steadily get worse as time goes on.
“It's horrible having Parkinson's,” says Taylor, a data analyst, now 41. “It limits my ability to be the dad and husband that I want to be in many cruel and debilitating ways.”
Today, more than 10 million people worldwide live with Parkinson's. Most are diagnosed when they're considerably older than Taylor, after age 60. Although recent research has called into question certain aspects of the disease’s origins, Parkinson’s eventually kills the nerve cells in the brain that produce dopamine, a signaling chemical that carries messages around the body to control movement. Many patients have lost 60 to 80 percent of these cells by the time they are diagnosed.
For years, there's been little improvement in the standard treatment. Patients are typically given the drug levodopa, a chemical that's absorbed by the brain’s nerve cells, or neurons, and converted into dopamine. This drug addresses the symptoms but has no impact on the course of the disease as patients continue to lose dopamine producing neurons. Eventually, the treatment stops working effectively.
BlueRock Therapeutics, a cell therapy company based in Massachusetts, is taking a different approach by focusing on the use of stem cells, which can divide into and generate new specialized cells. The company makes the dopamine-producing cells that patients have lost and inserts these cells into patients' brains. “We have a disease with a high unmet need,” says Ahmed Enayetallah, the senior vice president and head of development at BlueRock. “We know [which] cells…are lost to the disease, and we can make them. So it really came together to use stem cells in Parkinson's.”
In a phase 1 research trial announced late last month, patients reported that their symptoms had improved after a year of treatment. Brain scans also showed an increased number of neurons generating dopamine in patients’ brains.
Increases in dopamine signals
The recent phase 1 trial focused on deploying BlueRock’s cell therapy, called bemdaneprocel, to treat 12 patients suffering from Parkinson’s. The team developed the new nerve cells and implanted them into specific locations on each side of the patient's brain through two small holes in the skull made by a neurosurgeon. “We implant cells into the places in the brain where we think they have the potential to reform the neural networks that are lost to Parkinson's disease,” Enayetallah says. The goal is to restore motor function to patients over the long-term.
Five patients were given a relatively low dose of cells while seven got higher doses. Specialized brain scans showed evidence that the transplanted cells had survived, increasing the overall number of dopamine producing cells. The team compared the baseline number of these cells before surgery to the levels one year later. “The scans tell us there is evidence of increased dopamine signals in the part of the brain affected by Parkinson's,” Enayetallah says. “Normally you’d expect the signal to go down in untreated Parkinson’s patients.”
"I think it has a real chance to reverse motor symptoms, essentially replacing a missing part," says Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh.
The team also asked patients to use a specific type of home diary to log the times when symptoms were well controlled and when they prevented normal activity. After a year of treatment, patients taking the higher dose reported symptoms were under control for an average of 2.16 hours per day above their baselines. At the smaller dose, these improvements were significantly lower, 0.72 hours per day. The higher-dose patients reported a corresponding decrease in the amount of time when symptoms were uncontrolled, by an average of 1.91 hours, compared to 0.75 hours for the lower dose. The trial was safe, and patients tolerated the year of immunosuppression needed to make sure their bodies could handle the foreign cells.
Claire Bale, the associate director of research at Parkinson's U.K., sees the promise of BlueRock's approach, while noting the need for more research on a possible placebo effect. The trial participants knew they were getting the active treatment, and placebo effects are known to be a potential factor in Parkinson’s research. Even so, “The results indicate that this therapy produces improvements in symptoms for Parkinson's, which is very encouraging,” Bale says.
Tilo Kunath, a professor of regenerative neurobiology at the University of Edinburgh, also finds the results intriguing. “I think it's excellent,” he says. “I think it has a real chance to reverse motor symptoms, essentially replacing a missing part.” However, it could take time for this therapy to become widely available, Kunath says, and patients in the late stages of the disease may not benefit as much. “Data from cell transplantation with fetal tissue in the 1980s and 90s show that cells did not survive well and release dopamine in these [late-stage] patients.”
Searching for the right approach
There's a long history of using cell therapy as a treatment for Parkinson's. About four decades ago, scientists at the University of Lund in Sweden developed a method in which they transferred parts of fetal brain tissue to patients with Parkinson's so that their nerve cells would produce dopamine. Many benefited, and some were able to stop their medication. However, the use of fetal tissue was highly controversial at that time, and the tissues were difficult to obtain. Later trials in the U.S. showed that people benefited only if a significant amount of the tissue was used, and several patients experienced side effects. Eventually, the work lost momentum.
“Like many in the community, I'm aware of the long history of cell therapy,” says Taylor, the patient living with Parkinson's. “They've long had that cure over the horizon.”
In 2000, Lorenz Studer led a team at the Memorial Sloan Kettering Centre, in New York, to find the chemical signals needed to get stem cells to differentiate into cells that release dopamine. Back then, the team managed to make cells that produced some dopamine, but they led to only limited improvements in animals. About a decade later, in 2011, Studer and his team found the specific signals needed to guide embryonic cells to become the right kind of dopamine producing cells. Their experiments in mice, rats and monkeys showed that their implanted cells had a significant impact, restoring lost movement.
Studer then co-founded BlueRock Therapeutics in 2016. Forming the most effective stem cells has been one of the biggest challenges, says Enayetallah, the BlueRock VP. “It's taken a lot of effort and investment to manufacture and make the cells at the right scale under the right conditions.” The team is now using cells that were first isolated in 1998 at the University of Wisconsin, a major advantage because they’re available in a virtually unlimited supply.
Other efforts underway
In the past several years, University of Lund researchers have begun to collaborate with the University of Cambridge on a project to use embryonic stem cells, similar to BlueRock’s approach. They began clinical trials this year.
A company in Japan called Sumitomo is using a different strategy; instead of stem cells from embryos, they’re reprogramming adults' blood or skin cells into induced pluripotent stem cells - meaning they can turn into any cell type - and then directing them into dopamine producing neurons. Although Sumitomo started clinical trials earlier than BlueRock, they haven’t yet revealed any results.
“It's a rapidly evolving field,” says Emma Lane, a pharmacologist at the University of Cardiff who researches clinical interventions for Parkinson’s. “But BlueRock’s trial is the first full phase 1 trial to report such positive findings with stem cell based therapies.” The company’s upcoming phase 2 research will be critical to show how effectively the therapy can improve disease symptoms, she added.
The cure over the horizon
BlueRock will continue to look at data from patients in the phase 1 trial to monitor the treatment’s effects over a two-year period. Meanwhile, the team is planning the phase 2 trial with more participants, including a placebo group.
For patients with Parkinson’s like Martin Taylor, the therapy offers some hope, though Taylor recognizes that more research is needed.

BlueRock Therapeutics
“Like many in the community, I'm aware of the long history of cell therapy,” he says. “They've long had that cure over the horizon.” His expectations are somewhat guarded, he says, but, “it's certainly positive to see…movement in the field again.”
"If we can demonstrate what we’re seeing today in a more robust study, that would be great,” Enayetallah says. “At the end of the day, we want to address that unmet need in a field that's been waiting for a long time.”
Editor's note: The company featured in this piece, BlueRock Therapeutics, is a portfolio company of Leaps by Bayer, which is a sponsor of Leaps.org. BlueRock was acquired by Bayer Pharmaceuticals in 2019. Leaps by Bayer and other sponsors have never exerted influence over Leaps.org content or contributors.