Nobel Prize goes to technology for mRNA vaccines

Katalin Karikó, pictured, and Drew Weissman won the Nobel Prize for advances in mRNA research that led to the first Covid vaccines.
When Drew Weissman received a call from Katalin Karikó in the early morning hours this past Monday, he assumed his longtime research partner was calling to share a nascent, nagging idea. Weissman, a professor of medicine at the Perelman School of Medicine at the University of Pennsylvania, and Karikó, a professor at Szeged University and an adjunct professor at UPenn, both struggle with sleep disturbances. Thus, middle-of-the-night discourses between the two, often over email, has been a staple of their friendship. But this time, Karikó had something more pressing and exciting to share: They had won the 2023 Nobel Prize in Physiology or Medicine.
The work for which they garnered the illustrious award and its accompanying $1,000,000 cash windfall was completed about two decades ago, wrought through long hours in the lab over many arduous years. But humanity collectively benefited from its life-saving outcome three years ago, when both Moderna and Pfizer/BioNTech’s mRNA vaccines against COVID were found to be safe and highly effective at preventing severe disease. Billions of doses have since been given out to protect humans from the upstart viral scourge.
“I thought of going somewhere else, or doing something else,” said Katalin Karikó. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
Unlocking the power of mRNA
Weissman and Karikó unlocked mRNA vaccines for the world back in the early 2000s when they made a key breakthrough. Messenger RNA molecules are essentially instructions for cells’ ribosomes to make specific proteins, so in the 1980s and 1990s, researchers started wondering if sneaking mRNA into the body could trigger cells to manufacture antibodies, enzymes, or growth agents for protecting against infection, treating disease, or repairing tissues. But there was a big problem: injecting this synthetic mRNA triggered a dangerous, inflammatory immune response resulting in the mRNA’s destruction.
While most other researchers chose not to tackle this perplexing problem to instead pursue more lucrative and publishable exploits, Karikó stuck with it. The choice sent her academic career into depressing doldrums. Nobody would fund her work, publications dried up, and after six years as an assistant professor at the University of Pennsylvania, Karikó got demoted. She was going backward.
“I thought of going somewhere else, or doing something else,” Karikó told Stat in 2020. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
A tale of tenacity
Collaborating with Drew Weissman, a new professor at the University of Pennsylvania, in the late 1990s helped provide Karikó with the tenacity to continue. Weissman nurtured a goal of developing a vaccine against HIV-1, and saw mRNA as a potential way to do it.
“For the 20 years that we’ve worked together before anybody knew what RNA is, or cared, it was the two of us literally side by side at a bench working together,” Weissman said in an interview with Adam Smith of the Nobel Foundation.
In 2005, the duo made their 2023 Nobel Prize-winning breakthrough, detailing it in a relatively small journal, Immunity. (Their paper was rejected by larger journals, including Science and Nature.) They figured out that chemically modifying the nucleoside bases that make up mRNA allowed the molecule to slip past the body’s immune defenses. Karikó and Weissman followed up that finding by creating mRNA that’s more efficiently translated within cells, greatly boosting protein production. In 2020, scientists at Moderna and BioNTech (where Karikó worked from 2013 to 2022) rushed to craft vaccines against COVID, putting their methods to life-saving use.
The future of vaccines
Buoyed by the resounding success of mRNA vaccines, scientists are now hurriedly researching ways to use mRNA medicine against other infectious diseases, cancer, and genetic disorders. The now ubiquitous efforts stand in stark contrast to Karikó and Weissman’s previously unheralded struggles years ago as they doggedly worked to realize a shared dream that so many others shied away from. Katalin Karikó and Drew Weissman were brave enough to walk a scientific path that very well could have ended in a dead end, and for that, they absolutely deserve their 2023 Nobel Prize.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Electricity is emerging as a powerful treatment for chronic ailments.
Kelly, a case manager for an insurance company, spent years battling both migraines and Crohn's, a disease in which the immune system attacks the intestines.
For many people, like Kelly, a stronger electric boost to the vagus nerve could be life-changing.
After she had her large intestine removed, her body couldn't absorb migraine medication. Last year, about twice a month, she endured migraines so bad she couldn't function. "It would go up to a ten, and I would rock, wait it out," she said. The pain might last for three days.
Then her neurologist showed her a new device, gammaCore, that tames migraines by stimulating a nerve—not medication. "I don't have to put a chemical in my body," she said. "I was thrilled."
At first, Kelly used the device at the onset of a migraine, applying electricity to her pulse at the front of her neck for six minutes. The pain peaked at about half the usual intensity--low enough, she said, that she could go to work. Four months ago, she began using the device for two minutes each night as prevention, and she hasn't had a serious migraine since.
The Department of Defense and Veterans Administration now offer gammaCore to patients, but it hasn't yet been approved by Medicare, Medicaid, or most insurers. A month of therapy costs $600 before insurance or a generous financial assistance program kicks in.

A patient uses gammaCore, a non invasive vagal nerve stimulator device that was FDA approved in November 2018, to treat her migraine.
(Photo captured from a patient video at gammacore.com)
If the poet Walt Whitman wrote "I Sing The Body Electric" today, he might get specific and point to the vagus nerve, a bundle of fibers that run from the brainstem down the neck to the heart and gut. Singing stimulates it—and for many people, like Kelly, a stronger electric boost to the nerve could be life-changing.
The mind-body connection isn't just an idea — the vagus nerve literally carries signals from the mind to the body and back. It may explain the link between childhood trauma and illnesses such as chronic pain and headaches in adults. "How is it possible that a psychological event causes pain years later?" asked Peter Staats, co-founder of electroCore, which has won approval for its new device from the Food and Drug Administration (FDA) for both migraine and cluster headaches. "There has to be a mind-body interface, and that is the vagus nerve," he said.
Scientists knew that this nerve controlled your heart rate and blood pressure, but in the past decade it has been linked to both pain and the immune system.
"Everything is gated through the vagus -- problems with the gut, the heart, and the lungs," said Chris Wilson, a researcher at Loma Linda University, in California. Wilson is studying how vagus nerve stimulation (VNS) could help pre-term babies who develop lung infections. "Nearly every one of our chronic diseases, including cancer, Alzheimer's, Parkinson's, chronic arthritis and rheumatoid arthritis, and depression and chronic pain…could benefit from an appropriate stimulator," he said.
It's unfortunate that Kelly got her device only after her large intestine was gone. SetPoint Medical, a privately held California company founded to develop electronic treatments for chronic autoimmune diseases, has announced early positive results with VNS for both Crohn's and rheumatoid arthritis.
As SetPoint's chief medical officer, David Chernoff, put it, "We're hacking into the nervous system to activate a system that is already there," an approach that, he said, could work "on many diseases that are pain- and inflammation-based." Inflammation plays a role in much modern illness, including depression and obesity. The FDA already has approved VNS for both, using surgically implanted devices similar to pacemakers. (GammaCore is external.)
The history of VNS implants goes back to 1997, when the FDA approved one for treating epilepsy and researchers noticed that it rapidly lifted depression in epileptic patients. By 2005, the agency had approved an implant for treatment-resistant depression. (Insurance companies declined to reimburse the approach and it didn't take off, but that might change: in February, the Center for Medicare and Medicaid Services asked for more data to evaluate coverage.) In 2015, the FDA approved an implant in the abdomen to regulate appetite signals and help obese people lose weight.
The link to inflammation had emerged a decade earlier, when researchers at the Feinstein Institute for Medical Research, in Manhasset, New York, demonstrated that stimulating the nerve with electricity in rats suppressed the production of cytokines, a signaling protein important in the immune system. The researchers developed a concept of a hard-wired pathway, through the vagus nerve, between the immune and nervous system. That pathway, they argued, regulates inflammation. While other researchers argue that VNS is helpful by other routes, there is clear evidence that, one way or another, it does affect immunity.
At the same time, investors are seeking alternatives to drugs.
The Feinstein rat research concluded that it took only a minute a day of stimulation and tiny amounts of energy to activate an anti-inflammatory reflex. This means you can use devices "the size of a coffee bean," said Chernoff, much less clunky than current pacemakers—and advances in electronic technology are making them possible.
At the same time, investors are seeking alternatives to drugs. "There's been a push back on drug pricing," noted Lisa Rhoads, a managing director at Easton Capital Investment Group, in New York, which supported electroCore, "and so many unintended consequences."
In 2016, the U.S. National Institutes of Health began pumping money into relevant research, in a program called "Stimulating Peripheral Activity to Relieve Conditions," which focuses on "understanding peripheral nerves — nerves that connect the brain and spinal cord to the rest of the body — and how their electrical signals control internal organ function."
GlaxoSmithKline formed Galvani Bioelectronics with Google to study miniature implants. It had already invested in Action Potential Venture Capital, in Cambridge, Massachusetts, which holds SetPoint and seven other companies "that are all targeting a nerve to treat a chronic disease," noted partner Imran Eba. "I see a future in which bioelectronics medicine is competing directly with drugs," he said.
Treating the body with electricity could bring more ease and lower costs. Many people with serious auto-immune disease, for example, have to inject themselves with drugs that cost $60,000 a year. SetPoint's implant would cost less and only need charging once a week, using a charger worn around the neck, Chernoff said. The company receives notices remotely and can monitor compliance.
Implants also allow the treatment to target a nerve precisely, which could be important with Parkinson's, chronic pain, and depression, observed James Cavuoto, editor and publisher of Neurotech Reports. They may also allow for more fine-turning. "In general, the industry is looking for signals, biomarkers that indicate when is the right time to turn on and turn off the stimulation. It could dramatically increase the effectiveness of the therapy and conserve battery life," he said.
Eventually, external devices could receive data from biomarkers as well. "It could be something you wear on your wrist," Cavuoto noted. Bluetooth-enabled devices could communicate with phones or laptops for data capture. External devices don't require surgery and put the patient in charge. "In the future you'll see more customer specification: Give the patient a tablet or phone app that lets them track and modify their parameters, within a range. With digital devices we have an enormous capability to customize therapies and collect data and get feedback that can be fed back to the clinician," Cavuoto said.
Slow deep breathing, the traditional mind-body intervention, is "like watching Little League. What we're doing is Major League."
It's even possible to stimulate the vagus through the ear, where one branch of the bundle of fibers begins. In a fetus, the tissue that becomes the ear is also part of the vagus nerve, and that one bit remains. "It's the same point as the acupuncture point," explained Mark George, a psychiatrist and pioneer researcher in depression at Medical University of South Carolina in Charleston. "Acupuncture figured out years ago by trial and error what we're just learning about now."
Slow deep breathing, the traditional mind-body intervention, also affects the vagus nerve in positive ways, but gently. "That's like watching Little League," Staats, the co-founder of electroCore, said. "What we're doing is Major League."
In ten years, researcher Wilson suggested, you could be wearing "a little ear cuff" that monitors your basic autonomic tone, a heart-attack risk measure governed in part by the vagus nerve. If your tone looked iffy, the stimulator would intervene, he said, "and improve your mood, cognition, and health."
In the meantime, we can take some long slow breaths, read Whitman, and sing.
“Siri, Read My Mind”: A New Device Lets Users Think Commands
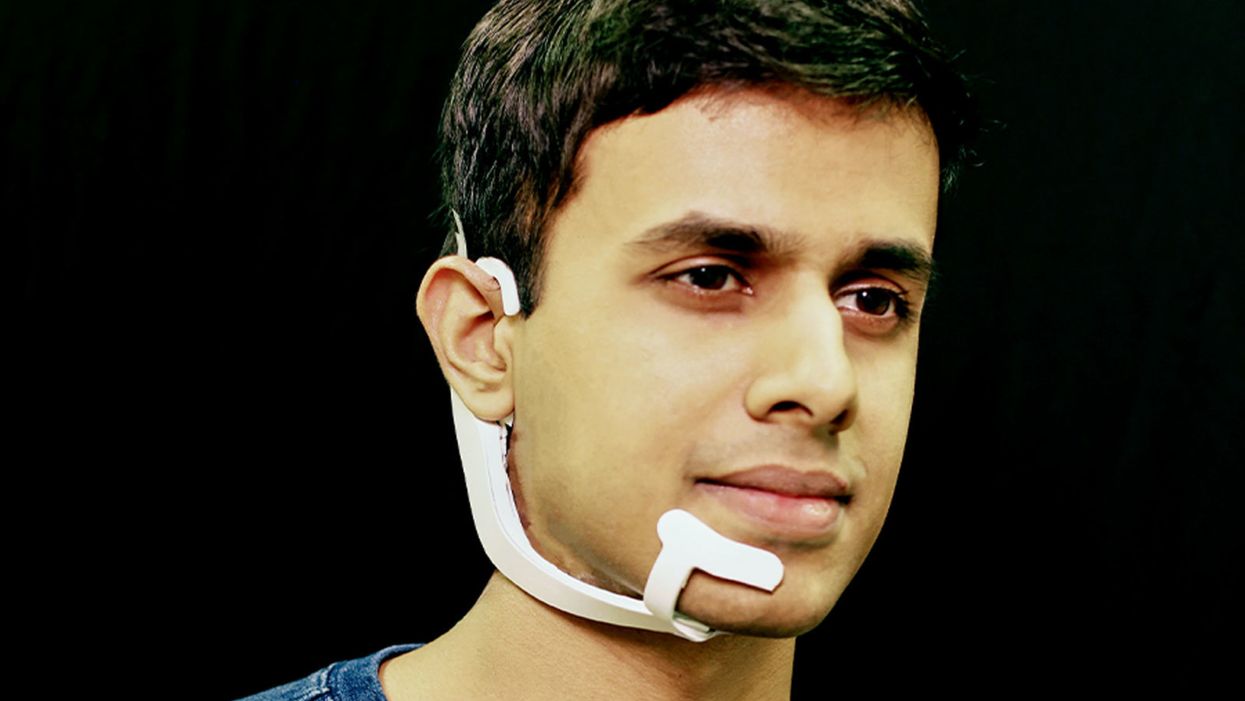
Arnav Kapur, a researcher in the Fluid Interfaces Group at MIT, wears an earlier prototype of the AlterEgo. A newer version is more discreet.
Sometime in the near future, we won't need to type on a smartphone or computer to silently communicate our thoughts to others.
"We're moving as fast as possible to get the technology right, to get the ethics right, to get everything right."
In fact, the devices themselves will quietly understand our intentions and express them to other people. We won't even need to move our mouths.
That "sometime in the near future" is now.
At the recent TED Conference, MIT student and TED Fellow Arnav Kapur was onstage with a colleague doing the first live public demo of his new technology. He was showing how you can communicate with a computer using signals from your brain. The usually cool, erudite audience seemed a little uncomfortable.
"If you look at the history of computing, we've always treated computers as external devices that compute and act on our behalf," Kapur said. "What I want to do is I want to weave computing, AI and Internet as part of us."
His colleague started up a device called AlterEgo. Thin like a sticker, AlterEgo picks up signals in the mouth cavity. It recognizes the intended speech and processes it through the built-in AI. The device then gives feedback to the user directly through bone conduction: It vibrates your inner ear drum and gives you a response meshing with your normal hearing.
Onstage, the assistant quietly thought of a question: "What is the weather in Vancouver?" Seconds later, AlterEgo told him in his ear. "It's 50 degrees and rainy here in Vancouver," the assistant announced.
AlterEgo essentially gives you a built-in Siri.
"We don't have a deadline [to go to market], but we're moving as fast as possible to get the technology right, to get the ethics right, to get everything right," Kapur told me after the talk. "We're developing it both as a general purpose computer interface and [in specific instances] like on the clinical side or even in people's homes."
Nearly-telepathic communication actually makes sense now. About ten years ago, the Apple iPhone replaced the ubiquitous cell phone keyboard with a blank touchscreen. A few years later, Google Glass put computer screens into a simple lens. More recently, Amazon Alexa and Microsoft Cortana have dropped the screen and gone straight for voice control. Now those voices are getting closer to our minds and may even become indistinguishable in the future.
"We knew the voice market was growing, like with getting map locations, and audio is the next frontier of user interfaces," says Dr. Rupal Patel, Founder and CEO of VocalID. The startup literally gives voices to the voiceless, particularly people unable to speak because of illness or other circumstances.
"We start with [our database of] human voices, then train our deep learning technology to learn the pattern of speech… We mix voices together from our voice bank, so it's not just Damon's voice, but three or five voices. They are different enough to blend it into a voice that does not exist today – kind of like a face morph."
The VocalID customer then has a voice as unique as he or she is, mixed together like a Sauvignon blend. It is a surrogate voice for those of us who cannot speak, just as much as AlterEgo is a surrogate companion for our brains.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon."
Voice equality will become increasingly important as Siri, Alexa and voice-based interfaces become the dominant communication method.
It may feel odd to view your voice as a privilege, but as the world becomes more voice-activated, there will be a wider gap between the speakers and the voiceless. Picture going shopping without access to the Internet or trying to eat healthily when your neighborhood is a food desert. And suffering from vocal difficulties is more common than you might think. In fact, according to government statistics, around 7.5 million people in the U.S. have trouble using their voices.
While voice communication appears to be here to stay, at least for now, a more radical shift to mind-controlled communication is not necessarily inevitable. Tech futurist Wagner James Au, for one, is dubious.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon. Generation Z has grown up with smartphones and games like Fortnite, so I don't see them quickly switching to a new form factor. It's still unclear if even head-mounted AR/VR displays will see mass adoption, and mind-reading devices are a far greater physical imposition on the user."
How adopters use the newest brain impulse-reading, voice-altering technology is a much more complicated discussion. This spring, a video showed U.S. House Speaker Nancy Pelosi stammering and slurring her words at a press conference. The problem is that it didn't really happen: the video was manufactured and heavily altered from the original source material.
So-called deepfake videos use computer algorithms to capture the visual and vocal cues of an individual, and then the creator can manipulate it to say whatever it wants. Deepfakes have already created false narratives in the political and media systems – and these are only videos. Newer tech is making the barrier between tech and our brains, if not our entire identity, even thinner.
"Last year," says Patel of VocalID, "we did penetration testing with our voices on banks that use voice control – and our generation 4 system is even tricky for you and me to identify the difference (between real and fake). As a forward-thinking company, we want to prevent risk early on by watermarking voices, creating a detector of false voices, and so on." She adds, "The line will become more blurred over time."
Onstage at TED, Kapur reassured the audience about who would be in the driver's seat. "This is why we designed the system to deliberately record from the peripheral nervous system, which is why the control in all situations resides with the user."
And, like many creators, he quickly shifted back to the possibilities. "What could the implications of something like this be? Imagine perfectly memorizing things, where you perfectly record information that you silently speak, and then hear them later when you want to, internally searching for information, crunching numbers at speeds computers do, silently texting other people."
"The potential," he concluded, "could be far-reaching."